8-K: Current report filing
Published on February 18, 2025
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported):
(Exact Name of Registrant as Specified in Charter)
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
||
| (Address of Principal Executive Offices) | (Zip Code) |
(
(Registrant’s telephone number, including area code)
Unite Acquisition 1 Corp.
12 E. 49th Street, 11th Floor, New York, NY 10017
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act: None.
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
EXPLANATORY NOTE
As used in this Current Report on Form 8-K (this “Report”), unless otherwise stated or the context clearly indicates otherwise, the terms the “Company,” “we,” “us” and “our” refer to Adaptin Bio, Inc., incorporated in the State of Delaware, and its subsidiaries after giving effect to the Merger (as defined below) and the company name change described herein.
The registrant was incorporated as Unite Acquisition 1 Corp. (“Unite Acquisition”) in the State of Delaware on March 10, 2022. Prior to the Merger (as defined below), the registrant was a “shell company” (as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)).
On February 11, 2025, Unite Acquisition’s wholly-owned subsidiary, Adaptin Acquisition Co., a Delaware corporation formed in the State of Delaware on January 30, 2025 (“Merger Sub”), merged with and into Adaptin Bio, Inc., a privately held Delaware corporation (“Private Adaptin”) formerly known as Centaur Bio Inc. Pursuant to this transaction (the “Merger”), Private Adaptin was the surviving corporation and became the Company’s wholly owned subsidiary, and all of the outstanding stock of Private Adaptin was converted into shares of the combined Company’s common stock, par value $0.0001 per share (the “Common Stock”). In addition, in connection with the Merger, all of Private Adaptin’s outstanding convertible promissory notes converted into shares of Common Stock at $3.30 per share and all of Private Adaptin’s outstanding warrants became exercisable for shares of Common Stock.
As a result of the Merger, we acquired the business of Private Adaptin and will continue its business operations as a public reporting company under the same name, Adaptin Bio, Inc. Concurrent with the consummation of the Merger, Private Adaptin changed its name to “Adaptin Bio Operating Corporation.”
Concurrent with the closing of the Merger, the Company also sold 1,080,814 units (the “Units”) at a purchase price of $4.40 per Unit, each consisting of (i) one share of Common Stock, (ii) a one-year warrant to purchase one share of Common Stock at an exercise price of $4.40 per share, and (iii) a five-year warrant to purchase one-half of a share of Common Stock at an exercise price of $6.60 per share. Additional information concerning the private placement is presented below under Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—The Offering” and under Item 3.02, “Unregistered Sales of Equity Securities.” This Report is not a solicitation for or an offer to purchase Units.
In accordance with “reverse merger” or “reverse acquisition” accounting treatment, our historical financial statements as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of Private Adaptin prior to the Merger, in all future filings with the U.S. Securities and Exchange Commission (the “SEC”).
This Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, which are filed as exhibits hereto and incorporated herein by reference.
This Report responds to the following Items:
| Item 1.01 | Entry into a Material Definitive Agreement. |
| Item 2.01 | Completion of Acquisition or Disposition of Assets. |
| Item 3.02 | Unregistered Sales of Equity Securities. |
| Item 3.03 | Material Modification to Rights of Security Holders. |
| Item 4.01 | Changes in Registrant’s Certifying Accountant. |
| Item 5.01 | Changes in Control of Registrant. |
| Item 5.02 | Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers. |
| Item 5.03 | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. |
| Item 5.05 | Amendments to the Registrant’s Code of Ethics, or Waiver of a Provision of the Code of Ethics. |
| Item 5.06 | Change in Shell Company Status. |
| Item 9.01 | Financial Statements and Exhibits. |
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act). As a result of the Merger, we have ceased to be a “shell company.” The information included in this Report constitutes the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act of 1933, as amended (the “Securities Act”).
FORWARD-LOOKING STATEMENTS
This Report, including the sections entitled “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Description of Business”, includes forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. Forward-looking statements relate to, among others, our plans, objectives and expectations for our business, operations and financial performance and condition, and can be identified by terminology such as “may,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “will,” “could,” “project,” “target,” “potential,” “continue” and similar expressions that do not relate solely to historical matters. Forward-looking statements are based on management’s belief and assumptions and on information currently available to management. Although we believe that the expectations reflected in forward-looking statements are reasonable, such statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by forward-looking statements.
Forward-looking statements include, but are not limited to, statements about:
| ● | our ability to raise additional money to fund our operations for at least the next twelve months as a going concern; |
| ● | our ability to develop our current and any future product candidates; |
| ● | our ability to receive marketing approval from the FDA for our product candidates; |
| ● | our ability to maintain our license rights to our intellectual property and to adequately protect or enforce our intellectual property rights; |
| ● | our reliance on third parties to supply drug substance and drug product for our clinical trials and preclinical studies, and produce commercial supplies of product candidates; |
| ● | our ability to market and commercialize our products, if approved; |
| ● | our product candidates’ ability to achieve market acceptance, if approved; |
| ● | developments and projections relating to our competitors and our industry; |
| ● | our ability to adequately control the costs associated with our operations; |
| ● | our dependence on third-party reimbursement for commercial viability; |
| ● | the impact of current and future laws and regulations, especially those related to drug development and drug pricing controls; |
| ● | potential cybersecurity risks to our operational systems, infrastructure, and integrated software by us or third-party vendors; |
| ● | the development of a market for our Common Stock; and |
| ● | other risks and uncertainties, including those listed under the caption “Risk Factors.” |
We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, operating results, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in the section titled “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions, the future events and trends discussed in this Report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
1
You should not rely upon forward-looking statements as predictions of future events. The events and circumstances reflected in the forward-looking statements may not be achieved or occur. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, performance, or achievements. We undertake no obligation to update any of these forward-looking statements for any reason after the date of this Report or to conform these statements to actual results or revised expectations, except as required by law.
You should read this Report and the documents that we reference in this Report as exhibits with the understanding that our actual future results, performance, and events and circumstances may be materially different from what we expect.
ITEM 1.01 ENTRY INTO A MATERIAL DEFINITIVE AGREEMENT.
The information contained in Item 2.01 below relating to the various agreements described therein is incorporated herein by reference. All descriptions of the agreements described below are qualified in their entirety by reference to the form of the relevant agreement that is filed as an exhibit to this Report and incorporated herein by reference.
ITEM 2.01 COMPLETION OF ACQUISITION OR DISPOSITION OF ASSETS.
THE MERGER AND RELATED TRANSACTIONS
Bridge Financings
Private Adaptin raised bridge financing through the offer and sale (a) in 2023 of $500,000 principal amount of its 10% Secured Promissory Notes (the “2023 Bridge Notes”) (including warrants to purchase up to 56,815 shares of our Common Stock at an exercise price of $4.40 per share) and (b) in 2024 of $1,000,000 principal amount of its 10% Secured Subordinated Convertible Promissory Notes (the “2024 Bridge Notes”, which in each case were sold to a limited number of accredited investors pursuant to Regulation D under the Securities Act. The 2023 Bridge Notes were exchanged for $500,000 principal amount of the Company’s 10% Secured Convertible Promissory Notes (the “Exchange Notes”). In connection with the note exchange, the holders of the 2023 Bridge Notes were also issued warrants to purchase up to 75,755 shares of our Common Stock at an exercise price of $3.30 per share. The Exchange Notes and the 2024 Bridge Notes are referred to herein as the “Bridge Notes.”
At the closing of the Offering (as defined below), the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, automatically converted into shares of Company common stock, par value $0.0001 per share (“Common Stock”), at a conversion price of $3.30 per share, or 501,140 shares of Company Common Stock (the “Note Conversion Shares”), and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of either $3.30 or $4.40 per share and with a term of five years, as described above (the “Pre-Merger Warrants”).
Merger Agreement
On February 11, 2025, we entered into the Merger Agreement with Merger-Sub and Private Adaptin, pursuant to which Merger Sub merged with and into Private Adaptin, with Private Adaptin continuing as the surviving corporation and as our wholly owned subsidiary.
Pursuant to the Merger Agreement, all of the outstanding capital stock of Private Adaptin was cancelled in exchange for shares of Common Stock, and all of the outstanding Private Adaptin warrants were assumed by, the Company at the, with appropriate adjustments to the per share exercise or conversion price thereof, and otherwise on their original terms and conditions. The total number of shares of Common Stock issued to pre-Merger stockholders of Private Adaptin was 3,249,999 shares.
2
Prior to the closing of the Offering (as defined below), Unite Acquisition’s board of directors adopted an equity incentive plan reserving a number of shares of Common Stock equal to 15% of the shares to be outstanding upon each closing of the Offering, up to a maximum aggregate amount of 15% of the fully diluted shares outstanding of the Company following the final closing of the Offering (assuming exercise or conversion of all then-outstanding Common Stock equivalents), for the future issuance, at the discretion of the board of directors, of options and other incentive awards to officers, key employees, consultants and directors of the Company and its subsidiaries. See “Compensation of Directors and Executive Officers—Description of the 2025 Equity Incentive Plan” below for more information about the equity incentive plan.
The sole holder of common stock of Unite Acquisition prior to the Merger, Lucius Partners, retained 3,250,000 shares of Common Stock after the Merger.
The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions.
As a condition to the Merger, we entered into a pre-Merger indemnity agreement with Unite Acquisition’s sole officer and director, Nathan P. Pereira, pursuant to which the Company agreed to indemnify Mr. Pereira for actions taken by him in his official capacity relating to the consideration, approval and consummation of the Merger and certain related transactions.
We expect the Merger to be treated as a recapitalization and reverse acquisition for us for financial reporting purposes. Private Adaptin is to be considered the acquirer for accounting purposes, and our historical financial statements before the Merger will be replaced with the historical financial statements of Private Adaptin before the Merger in future filings with the SEC. The Merger is intended to be treated as a tax-free reorganization under Section 368(a) of the Internal Revenue Code of 1986, as amended.
The Offering
Immediately following the effective time of the Merger, we sold in a closing of our private placement offering (the “Offering”), 1,080,814 Units, for an aggregate purchase price of $4,755,581.60, at a purchase price of $4.40 per Unit, with each Unit consisting of (i) one share of Common Stock, (ii) a warrant representing the right to purchase one share of Common Stock, exercisable from issuance until one year after the final closing of the Offering at an exercise price of $4.40 per share (the “A Warrant”), and (iii) a warrant, representing the right to purchase one-half of a share of Common Stock, exercisable from issuance until five years after the final closing of the Offering at an exercise price of $6.60 per whole share (the “B Warrant,” and together with the A Warrant, the “Warrants”) (such shares of Common Stock issuable upon the exercise of the Warrants, the “Warrant Shares”). The Company and Laidlaw & Company (UK) Ltd. (the “Placement Agent”) may conduct additional closings of the Offering at their discretion for up to $8.5 million (the “Maximum Offering”). Each investor in any subsequent closing will be required to represent that, as of the date of entering into the subscription agreement and the date of the applicable closing, it (i) has a substantive, pre-existing relationship with us or has direct contact with us or the Placement Agent outside of the Offering, and (ii) did not independently contact us or a Placement Agent or become interested in the Offering as a result of reading or otherwise being aware of this Current Report, any press release or any other public disclosure disclosing the terms of the Offering.
3
In connection with the Offering, the Placement Agent (a) will be paid at each closing from the Offering proceeds a total cash commission of 10.0% of the aggregate gross purchase price paid by purchasers in the Offering at that closing (the “Cash Fee”), (b) will be paid at each closing from the Offering proceeds a total non-allocable expense allowance equal to 2.0% of the aggregate gross purchase price paid by purchasers in the Offering at that Closing (the “Expense Allowance”), and (c) will receive (and/or its designees will receive) warrants to purchase a total number of shares of Common Stock equal to 10.0% of the sum of (i) the number of shares of Common Stock included in the Units sold in the Offering at that closing and (ii) the number of shares of Common Stock issuable upon exercise of the Warrants included in the Units sold in the Offering at that closing, with a term expiring seven years after the final closing date and an exercise price of $4.40 per share (the “Placement Agent Warrants”). Any sub-agent of the Placement Agent that introduces investors to the Offering will be entitled to share in the Cash Fee, Expense Allowance and Placement Agent Warrants attributable to those investors pursuant to the terms of an executed sub-agent agreement with such Placement Agent. The Company has agreed to pay certain other expenses of the Placement Agent, including the fees and expenses of its counsel, in connection with the Offering. Subject to certain customary exceptions, we will also indemnify the Placement Agent to the fullest extent permitted by law against certain liabilities that may be incurred in connection with the Offering, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agent and its sub-agents may be required to make in respect of such liabilities.
Description of Warrants
The A Warrants will have an exercise price of $4.40 per share and a term of one year from the final closing of the Offering and will be exercisable solely for cash.
The B Warrants will have an exercise price of $6.60 per share and a term of five years from the final closing of the Offering and will be exercisable either for cash or, when there is no effective registration statement covering the shares of Common Stock issuable upon exercise of the B Warrants, on a cashless net exercise basis.
The Warrants will have “weighted average” anti-dilution protection, subject to customary exceptions, including but not limited to issuances of awards under the executive incentive plan.
The Placement Agent Warrants will have an exercise price of $4.40 per share and a term of seven years from the final closing of the Offering and will be exercisable for cash or on a cashless net exercise basis.
Lock-Up Agreements
All officers and directors of the Company following the Merger and their affiliates and associated entities entered into lock-up agreements with us for a term ending two years after the closing of the Merger, whereby they have agreed to certain restrictions on the sale or disposition (including pledge) of all of our Common Stock held by (or issuable to) them. The lock-up agreements contain customary transfer exceptions.
Registration Rights
In connection with the Merger and the Offering, the Company entered into a registration rights agreement (the “Registration Rights Agreement”), pursuant to which the Company will file a registration statement (the “Registration Statement”) within 60 calendar days after termination of the Offering with the SEC registering for resale the following: (a) the shares of Common Stock issued pursuant to the subscription agreements in the Offering (the “Offering Shares”); (b) the Warrant Shares; (c) shares of Common Stock issued or issuable upon exercise of the Pre-Merger Warrants (the “Pre-Merger Warrant Shares”); (d) the Note Conversion Shares; (e) shares of Common Stock held by stockholders of the Company prior to the Merger and remaining outstanding immediately following the effective time of the Merger (the “Registrable Pre-Merger Shares”); (f) shares of Common Stock issued or issuable upon exercise of the Placement Agent Warrants (the “Placement Agent Warrant Shares”); and (g) other shares of restricted Common Stock held by the signatories to the Registration Rights Agreement acquired or issuable in respect of the foregoing shares of Common Stock by way of conversion, dividend, stock-split, distribution or exchange, merger, consolidation, recapitalization or reclassification or similar transaction (from (a) to (g), the “Registrable Shares”).
4
The 60 day period shall be tolled as of February 14, 2025, and resume when the audited financial statements of the Company for the fiscal year ending December 31, 2024 are issued, or March 31, 2025, whichever is earlier (the number of days of the 60 day period tolled as of February 14, 2025, the “Tolling Period”), and provided further that the Tolling Period, if applicable, shall be a minimum of 14 calendar days. The Company will use its commercially reasonable efforts to cause the Registration Statement to be declared effective within 120 calendar days after the termination of the Offering, plus, if applicable, a number of days equal to the Tolling Period.
If (a) the Company is late in filing the Registration Statement, (b) the Registration Statement is not declared effective within 120 days after the final closing date of the Offering (subject to certain exceptions), (c) the Registration Statement ceases for any reason to remain effective or the holders of Registrable Shares are otherwise not permitted to utilize the prospectus therein to resell the Registrable Shares for a period of more than five consecutive trading days (subject to permitted blackout periods (as defined in the Registration Rights Agreement) and certain other exceptions), or (d) following the listing or inclusion for quotation of the Common Stock on OTC Markets, Nasdaq, NYSE or NYSE American, the Registrable Shares are not listed or included for quotation on such a market, or trading of the Common Stock is suspended or halted on the principal market for the Common Stock, for more than three consecutive trading days (subject to certain exceptions) (each, a “Registration Event”), then in any such case monetary penalties payable by the Company to the holders of the Registrable Shares that are affected by such Registration Event will commence to accrue at a rate equal to 12% per annum of the total of the following, to the extent applicable to such holder: (i) if the holder is a holder of Note Conversion Shares, the product of $3.30 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Note Conversion Shares held by or issuable to such holder as of the date of such Registration Event, (ii) if the holder is a holder of Offering Shares, A Warrant Shares, Pre-Merger Warrant Shares, Registrable Pre-Merger Shares or Placement Agent Warrant Shares, the product of $4.40 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Offering Shares, A Warrant Shares, Pre-Merger Warrant Shares, Registrable Pre-Merger Shares or Placement Agent Warrant Shares, as the case may be, held by or issuable to such holder as of the date of such Registration Event, or (iii) if the holder is a holder of B Warrant Shares, the product of $6.60 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Note Conversion Shares held by or issuable to such holder as of the date of such Registration Event, but in each case, only with respect to such holder’s Registrable Shares that are affected by such Registration Event and only for the applicable Registration Default Period (as defined in the Registration Rights Agreement); provided, however, that in no event will the aggregate of any such penalties exceed 5% of the offering price per share. Notwithstanding the foregoing, no penalties will accrue with respect to any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of Common Stock which may be included in the Registration Statement (a “Cutback Comment”). Any cutback resulting from a Cutback Comment (the “Reduction Securities”) shall be in the following order: (i) first from the Placement Agent Warrant Shares, on a pro rata basis among the holders thereof; (ii) second from the Registrable Pre-Merger Shares, on a pro rata basis among the holders thereof; (iii) third from the Offering Shares, the Offering Warrant Shares, the Pre-Merger Warrant Shares and the Note Conversion Shares, on a pro rata basis among the holders thereof.
The Company will use its commercially reasonable efforts to keep the Registration Statement continuously effective until the earlier of five years from the date it is declared effective by the SEC or for such shorter period ending on the earlier to occur of (a) the sale of all Registrable Shares and (b) the availability of Rule 144 for the holders to sell all of the Registrable Shares without restrictions, including volume limitations, and without the need for current public information, required by Rule 144 (other than Rule 144(i)) or otherwise, during any 90-day period.
If fewer than all of the Registrable Shares are included in the Registration Statement when it becomes effective, the Company will use its commercially reasonable efforts within 60 calendar days after the effective date of the Registration Statement, or within ten business days after the first date that is permitted by the SEC to, register for resale as many of the Reduction Securities as the SEC will permit (pro rata among the holders of such Reduction Securities) using one or more registration statements that it is then entitled to use, and to cause such registration statement(s) to become effective as soon as practicable, until all of the Reduction Securities have been so registered; provided, however, that the Company shall not be required to register such Reduction Securities during a Blackout Period (as defined in the Registration Rights Agreement).
The holders of Registrable Shares shall have “piggyback” registration rights for Registrable Shares not registered as provided above with respect to any registration statement filed by the Company following the effectiveness of the aforementioned Registration Statement that would permit the inclusion of such underlying shares, subject, in an underwritten offering, to customary cut-back on a pro rata basis among the holders of Registrable Shares if the underwriter or the Company determines that marketing factors require a limitation on the number of shares of stock or other securities to be underwritten.
5
OTC Quotation
Our Common Stock is currently not listed on a national securities exchange or any other exchange, or quoted on an over-the-counter market. Following completion of the Offering, we intend to cause our Common Stock to be quoted on the OTC Markets QB tier as soon as practicable following the effectiveness of the Registration Statement. However, we cannot assure you that we will be able to do so and, even if we do so, there can be no assurance that our Common Stock will continue to be quoted on the OTC Markets or quoted or listed on any other market or exchange, or that an active trading market for our Common Stock will develop or continue. See “Risk Factors—There is currently no market for our Common Stock and there can be no assurance that any market will ever develop.”
Our 2025 Equity Incentive Plan
Pursuant to the Merger Agreement, Unite Acquisition adopted the 2025 Equity Incentive Plan (the “2025 Plan”), which provides for the issuance of incentive awards of stock options, restricted stock awards, restricted stock units, stock appreciation rights and performance awards. The number of shares reserved for issuance under the 2025 Plan will increase automatically on January 1 of each of 2026 through 2035 by the number of shares equal to the lesser of 4% of the total number of outstanding shares of our Common Stock as December 31 (calculated on a fully-diluted and as-converted basis), or a number as may be determined by the Company’s board of directors. See “Compensation of Directors and Executive Officers—Description of the 2025 Equity Incentive Plan” below for more information about the 2025 Plan.
Departure and Appointment of Directors and Officers
Prior to the effective time of the Merger, the Unite Acquisition board of directors consisted of one member, Mr. Pereira, who also was its President, Chief Executive Officer, Chief Financial Officer, and Secretary. As of the Effective Time, Mr. Pereira resigned from the board of directors, and Patrick Gallagher, Simon Pedder, Michael J. Roberts, J. Nick Riehle and Anthony Zook were appointed to the Company’s board of directors.
Also, as of the Effective Time, Mr. Pereira resigned from all officer positions with the Company, and Simon Pedder was appointed as our Executive Chairman, Michael J. Roberts was appointed as our President and Chief Executive Officer, Timothy L. Maness was appointed as our Chief Financial Officer, and L. Arthur Hewitt was appointed as our Chief Development Officer.
See “Management” below for information about our new directors and executive officers.
Combined Company Ownership
Following the Merger and the closing of the Offering, there are issued and outstanding 8,081,953 shares of our Common Stock, as follows:
| ● | the stockholders of Private Adaptin prior to the Merger will hold 3,249,999 shares of our Common Stock; |
| ● | investors in the Offering will hold 1,080,814 shares of our Common Stock; |
| ● | former holders of the Bridge Notes will hold 501,140 shares of our Common Stock; and |
| ● | Unite Acquisition’s sole stockholder prior to the Merger will hold 3,250,000 shares of our Common Stock. |
In addition, there are outstanding:
| ● | A Warrants to purchase 1,080,814 shares of our Common Stock; |
| ● | B Warrants to purchase 540,407 shares of our Common Stock; |
| ● | Placement Agent Warrants to purchase an aggregate of 270,204 shares of our Common Stock; and |
| ● | Pre-Merger Warrants to purchase 132,570 shares of our Common Stock. (Like the B Warrants, the Pre-Merger Warrants will be exercisable either for cash or, when there is no effective registration statement covering the shares of Common Stock issuable upon exercise of the Pre-Merger Warrants, on a cashless net exercise basis.) |
6
In addition, there are up to 2,196,390 shares of our Common Stock reserved for future issuance under the 2025 Plan. No other securities convertible into or exercisable or exchangeable for our Common Stock are outstanding as of the date of this Current Report.
Accounting Treatment; Change of Control
The Merger is being accounted for as a “reverse merger” or “reverse acquisition,” and Private Adaptin is deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that are reflected in our financial statements relating to periods prior to the Merger are those of Private Adaptin, and are recorded at the historical cost basis of Private Adaptin, and the consolidated financial statements after completion of the Merger will include the assets and liabilities of Private Adaptin, historical operations of Private Adaptin, and operations of the Company from the Closing Date. As a result of the issuance of the shares of our Common Stock pursuant to the Merger, a change in control of the Company occurred as of the date of consummation of the Merger.
Except as described in this Report, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our board of directors and, to our knowledge, no other arrangements exist that might result in a change of control of the Company.
We continue to be a “smaller reporting company,” as defined under the Exchange Act, and an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) following the Merger. We believe that as a result of the Merger, we have ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act).
FORM 10 INFORMATION
DESCRIPTION OF BUSINESS
Formation History
The Company is a Delaware corporation initially formed in March 2022 as Unite Acquisition 1 Corp. Effective February 11, 2025, Unite Acquisition’s wholly owned subsidiary, Merger Sub, merged with an into Private Adaptin, a Delaware corporation. Private Adaptin was the surviving corporation in the transaction and became Unite Acquisition’s wholly owned subsidiary, renamed as Adaptin Operating Co. At the same time, Unite Acquisition changed its name to Adaptin Bio, Inc.
The Company’s website address is www.adaptinbio.com and we can be contacted at info@adaptinbio.com. Information contained on, or that can be accessed through, our website is not a part of this Report.
Business Overview
Adaptin is a biopharmaceutical company pioneering a transformational approach to enhance the transfer of therapeutics into the brain, facilitating the treatment of brain cancers and other unmet medical conditions. The Company’s proprietary technology harnesses the human immune system’s ability to target, recognize, destroy or deliver therapeutics to specific cells, including cancer cells. Our mission is to be the global leader and pioneer of this new treatment paradigm, integrating recombinant technology, gene therapy and cell therapy to address the challenges of targeting and delivering effective therapies, including to the brain for cancer and other central nervous system (“CNS”) indications.
The cause(s), or etiology, of many diseases can be addressed in part through manipulation of engineered cells. We view targeted manipulation of the human immune system, together with recombinant technology and/or gene therapy, as a therapeutically disruptive transformation in the way we treat brain and other diseases. Our experienced group of scientists and business leaders are developing our proprietary in vivo and ex vivo technology platforms to revolutionize treatment across a broad array of therapeutic areas with unmet treatment needs, including oncology, CNS disorders, autoimmune disease and cardiovascular diseases, among others. Our lead product candidate has been recently accepted under an investigator-led IND to begin first-in-human studies in brain cancer. Our goal is to complete preclinical studies on additional product candidates and file multiple investigational new drug applications (each, an “IND”) in 2026, if not earlier.
7
Adaptin’s novel technology was originally developed by researchers in the Department of Neurosurgery at Duke University, led by Dr. John H. Sampson, the prior Robert H. and Gloria Wilkins, Distinguished Professor and Chair of the Department of Neurosurgery and currently the Dean and Vice Chancellor at the University of Colorado School of Medicine. The group recognized that adoptive transfer of specifically activated functional human immune cells significantly increases the “hitchhiking” and intracerebral accumulation of macromolecules that are bound to their surface. While circulating naïve T cells do not typically penetrate the CNS, activated T cells are known to traffic frequently past the blood brain barrier (“BBB”) and perform routine immune surveillance in the CNS. Adaptin and its collaborators at Duke University are taking advantage of this CNS trafficking to enhance the localization of macromolecules and other agents to the CNS for cancer and other CNS disorders.
Adaptin is closely working with researchers at Duke University to translate preclinical proof of concept data of its first proprietary platform technology called BRiTE (Brain Bispecific T-cell Engager) into human clinical trials. BRiTE focuses on the transport of difficult to deliver T cell targeting agents to tumor tissue, including in the immunoprivileged brain and overcoming the challenges with other immunotherapeutic approaches. BRiTE is a translatable method to specifically target malignant glioma using a tumor-specific, fully human bispecific antibody that redirects patients’ own T cells to recognize and destroy tumor cells.
The first application of Adaptin’s technology is APTN-101, a proprietary epidermal growth factor receptor variant III, or EGFRVIII, BRiTE in order to eliminate malignant glioma tumors in a variety of aggressive preclinical tumor models where the tumor is implanted behind the BBB in the CNS (i.e., orthotopic). We designed APTN-101 to specifically redirect T cells against tumors expressing a well-characterized, mutated form of epidermal growth factor receptors (“EGFRs”) known as EGFRVIII, on a number of tumor types, including glioblastoma, breast and lung cancer. Because EGFRVIII is exclusively expressed on tumor cells, but not normal healthy cells, we believe it represents an ideal target for immunotherapy. We have made significant progress towards first-in-human clinical studies, including:
| ● | A pre-IND meeting with the U.S. Food and Drug Administration (the “FDA”) outlining a clear path to filing an IND; |
| ● | Completion of single-dose IND-enabling preclinical studies; |
| ● | Submission in April 2023 of an IND for an investigator-initiated, single-dose clinical trial, and its acceptance in May 2023 by the FDA; and |
| ● | Manufacturing of APTN-101 in more than sufficient quantities for Phase 1 trials. |
We also expect to expand our proprietary platform to other targets and indications. The Company is exploring several external opportunities to continue to advance and expand the product pipeline.
Strategy
Our goal is to become a leading biopharmaceutical company focused on the transfer of drugs across barriers and to targeted tissues, including the brain and CNS, to transform current treatment paradigms for patients and address unmet medical needs. The critical components of our strategy are as follows:
| ● | Advance the development of APTN-101 for the treatment of glioblastoma. The FDA’s acceptance in May 2023 of the IND for APTN-101 for the treatment of glioblastoma sets the stage for first-in-human clinical trials. |
| ● | Advance preclinical development of APTN-101 to support one or more additional INDs for additional kinds of cancer. We have designed APTN-101 to incorporate EGFRvIII, which is expressed on a number of tumor types, including breast and lung cancer (with or without brain metastases), so we are considering pre-clinical work to support INDs for these indications to be filed in 2026, if not earlier. |
8
| ● | Design and advance other early-stage drug product candidates for undisclosed rare and unmet needs. Because our proprietary technology enables drugs to cross barriers and target tissues, including the brain, we believe it has numerous potential applications in areas of unmet medical need. We are evaluating which of those indications would be most strategic to pursue in the near-term, and plan to initiate one or more preclinical studies in 2025 to support the filing of future INDs. |
| ● | Acquire, in-license or develop complementary delivery technologies that will allow us to produce BRiTE compounds or manipulate and activate immune cells in vivo. We continually evaluate technologies that will further enhance therapeutic effect, improve safety and manufacturability, or reduce costs of our products. |
| ● | Acquire targeted clinical compounds for conditions with unmet needs where our technology could be transformative. We continually evaluate development and in-licensing opportunities and may acquire clinical compounds for conditions with unmet medical needs where our technology’s ability to cross barriers and target specific tissues, including the brain, could be transformative of the treatment paradigm. |
| ● | Pursue a capital-efficient commercialization strategy. For products with smaller and/or orphan patient populations, our plan is to build an infrastructure to commercialize our drug products within the United Sates. Drawing upon our experience in commercializing specialty pharmaceutical products, we aim to build a specialized yet efficient infrastructure that will support the entire commercialization continuum, including stakeholder education, treatment decision and initiation, and product access throughout the patient journey. In addition, we plan to seek established companies to commercialize our drug products for larger addressable markets and outside of the United States. |
| ● | Leverage, protect and enhance our intellectual property portfolio and secure patents for additional products and indications. We intend to expand our intellectual property, grounded in securing composition of matter and method of use patents for new products and indications. We plan to enhance the intellectual property portfolio further through learnings from ongoing preclinical studies, clinical trials and manufacturing processes. |
| ● | Outsource capital-intensive operations. We plan to continue to outsource capital-intensive operations, including most clinical development and all manufacturing operations of our product candidates, and to facilitate the rapid development of our pipeline by using high quality specialist vendors and consultants in a capital efficient manner. |
Glioblastoma
Background
Glioblastoma multiforme (“GBM”), the highest grade (WHO IV) astrocytoma, is the most common and malignant brain tumor, accounting for about 50% of all gliomas and 12%–15% of all brain tumors. GBM tumor cells, which arise from stem cells or immature astrocytes due to genetic abnormalities, grow rapidly and disseminate in the brain. In addition, GBM cells can invade the intracranial blood vessels to areas away from the tumor core.
Although glioblastoma can happen anywhere in the brain, it usually forms in the frontal lobe and the temporal lobe. Glioblastoma rarely occurs in the brain stem or spinal cord. As glioblastoma grows, it spreads into the surrounding brain. This makes it difficult to remove the entire tumor with surgery. Although radiation therapy and chemotherapy can reach the tumors, glioblastoma cells can survive and regrow. Glioblastoma is very challenging to treat due to tumor-specific features, such as its rapid growth rate, the poor function of the immune system cells within the tumor, and inherent resistance of the tumor cells to many types of treatments.
There is no direct risk factor associated with most cases of glioblastoma. Certain rare genetic diseases, such as Li-Fraumeni and Lynch syndrome, are associated with gliomas. However, these affect only a small portion of patients with glioblastoma. Besides genetic syndromes, the only well-established risk factor is prior exposure to ionizing radiation that is used to treat certain head and neck cancers.
9
Brain tumor symptoms vary and depend on the tumor location. The most common glioblastoma symptoms are headaches, seizures, and progressively worsening numbness or weakness. Headaches with red flag symptoms warrant a trip to the doctor for a neurologic evaluation. Red flag symptoms include waking up due to pain, worsening pain on changing position, and continuous pain not relieved with over-the-counter headache medications.
Neurologic imaging with an MRI of the brain is often the first step in diagnosis. Brain imaging showing contrast-enhancing masses can be suggestive of glioblastoma. Most cases can be definitively diagnosed after surgery through histological testing. This takes place when a neuropathologist examines tissue or cells under a microscope to help confirm a glioblastoma diagnosis.
Incidence and Mortality
In the United States, the average annual age-adjusted incidence of glioblastoma is 3.2 per 100,000 population (or about 12,000 patients annually) with an average age of 64 at diagnosis. Glioblastoma is 1.6 times more common in males compared with females and 2.0 times higher in Caucasians compared to African Americans, with lower incidence in Asians and American Indians. Globally, glioblastoma incidence is highest in North America, Australia, and Northern and Western Europe. Veterans who served in Iraq or Afghanistan are 26% more likely to develop glioblastoma, according to the U.S. Department of Veterans Affairs and National Institutes of Health data, likely due to environmental exposures. Malignant primary brain tumors are the most frequent cause of cancer death in children, are more common than Hodgkin lymphoma, ovarian and testicular cancer and are responsible for more deaths than malignant melanoma.
Overall, the one-year relative survival rate is about 40% for patients diagnosed in the United States. The five-year survival rate is only about 5%. Treatment outcome remains poor, with a median survival rate of about 15 months.
Current Treatment/Management
A patient’s care team will take into account age, functional status, medical history and medication tolerability when planning the best treatment. For most newly diagnosed patients, the standard approach utilizes what is known as the Stupp protocol. Treatment is comprised of maximal surgical resection, which allows for accurate histological diagnosis, tumor genotyping, and a reduction in tumor volume, followed by 6 weeks of radiotherapy and concomitant daily temozolomide and a further 6 cycles of maintenance temozolomide. In patients with minimal functional impairment, the median overall survival (“OS”) is 15 months for radiotherapy plus temozolomide versus 12 months for radiotherapy alone.
Treatment options in the relapsed or recurrent setting are less well defined, with no established standard of care and little evidence for any interventions that prolong OS. Indeed, a significant proportion of patients may not even be eligible for second-line therapy. Options include further surgical resection, reirradiation, systemic therapies such as carmustine or bevacizumab, combined approaches, or supportive care alone.
Patients may also be treated with tumor treatment fields. This is a portable device placed on the scalp that uses mild electrical fields to try to interrupt cancer cell growth.
Standard-of-care treatments fail to specifically eliminate tumor cells and are limited by incapacitating damage to surrounding normal brain and systemic tissues leading to lymphopenia and many other detrimental side effects. All of this demonstrates that a more targeted immunotherapeutic approach is needed.
10
Over the last decade, emerging immunotherapies (such as monoclonal antibodies, oncolytic virus therapy, adoptive cell therapy, and cellular vaccines therapy) aimed at improving specific immune response against tumor cells have brought a glimmer of hope to patients with GBM. Adoptive cell therapy, including tumor-infiltrate lymphocytes (“TILs”) transfer and genetically engineered T cells transfer, is one of the most significant breakthroughs in the field of immune-oncology. Chimeric antigen receptor (“CAR”) engineered autologous T cells have produced sustained remissions in refractory lymphomas, but this approach needs further study in the treatment of solid tumors. While there is significant potential with targeted immunotherapy in GBM, significant challenges remain, including primarily the difficulty of crossing the BBB.
Bi-Specific Antibodies
The concept of bispecific antibodies was first introduced in 1980s as a method to target multiple antigens by a single antibody. The recombinant bispecific antibodies are classified into two types. The first are antibodies containing the crystallizable region (i.e., Fc-containing antibodies) and the second are antibody derivatives without Fc regions. The Fc region is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. The mechanism of action of bispecific antibodies includes binding to the tumor cells on one side through the Fab (antigen-binding region) portion of the antibody against the tumor-specific antigen (such as CD19, HER2, EGFR, or GD2) and to the immune effector cells such as T cells and NK cells, which leads to activation of those immune effector cells and Fc-receptor bearing phagocytic cells such as monocytes/macrophages that can also mediate direct lysis of the tumor cells.
Bispecific antibodies termed bispecific T cell engagers (“BiTEs”) are monomeric proteins consisting of two antibody-derived single-chain variable fragments (“scFvs”) translated in tandem. These constructs possess one effector-binding arm specific for the epsilon subunit of T-cell CD3 and an opposing target-binding arm directed against an antigen that is expressed on the surface of tumor cells (e.g., EGFRvIII).
We believe EGFRvIII is an attractive target tumor specific antigen, in part because it is specific to cancer cells and is not expressed in non-tumor tissue. Importantly, antibodies directed against EGFRvIII are entirely tumor-specific and do not cross react with the wild-type receptor located on healthy cells. Therefore, by retargeting T cells against the tumor-specific EGFRvIII antigen, we believe we can avoid killing healthy tissue and the related adverse effects. EGFRs are involved in deregulated cancer signaling pathways, leading to atypical proliferation and growth of tumor cells. EGFRvIII is the most common variant not presented in a major histocompatibility complex (“MHC”) -dependent manner and is seen in approximately 31 to 50% of patients with GBM and in a broad array of other cancers including breast and lung carcinoma. Lung and breast carcinoma are the two main types of cancer that lead to secondary brain tumors (i.e., brain metastases) in about 25% of these patients. Among patients with EGFRvIII-positive GBM, 37 to 86% of tumor cells express the mutated receptor, indicating that the mutation is translated with significant consistency.
Among patients with GBM, expression of EGFRvIII is an independent, negative prognostic indicator. EGFRvIII also enhances the growth of neighboring EGFRvIII-negative tumor cells via cytokine-mediated paracrine signaling and by transferring a functionally active oncogenic receptor to EGFRvIII-negative cells through the release of lipid-raft related microvesicles. Recent research has also found that EGFRvIII is expressed in glioma stem cells, an important consideration given the paradigm that tumor stem cells represent a subpopulation of cells that give rise to all differentiated tumor cells. Altogether, the specificity, high frequency of surface expression and oncogenicity of the EGFRvIII mutation make it an ideal target for antibody-based immunotherapy.
The divalent structure of BiTEs brings T cells into close proximity to the tumor cell, creating a synapse. Following BiTE-mediated synapse formation, T cells proliferate, secrete pro-inflammatory cytokines and express surface activation markers. Following BiTE-mediated synapse formation, T cells release perforin and granzyme proteases that kill tumor cells. BiTEs are capable of mediating serial rounds of killing and can trigger specific tumor cell killing from naïve T cells at exceedingly low concentrations and effector-to-target ratios.
It is well established that certain gliomas, such as glioblastoma, are uniquely shielded from the immune system due to its location within the CNS. While this privilege is not absolute, a significant proportion of tumors have been noted to be devoid of any TILs that can be redirected by bispecific T-cell engagers. In those tumors that do demonstrate invasion by TILs, they are often induced to be dysfunctional and anergic by the suppressive tumor microenvironment. Increased numbers of intratumoral CD8+ cytotoxic T lymphocytes (“CTLs”) have been associated with favorable outcomes in patients with glioblastoma.
11
Concomitant administration of stimulated CTLs may therefore synergistically enhance the efficacy of this treatment. The migration of T-cell engagers across the BBB may also be facilitated by activated T cells which adhere to the brain microvascular endothelium and subsequently cross by diapedesis. Concurrent administration of activated functional T cells could therefore enhance the trafficking of bispecific T-cell engagers and other therapeutics into the intracranial compartment, increasing their density at the tumor site and thus the therapeutic effect.
Additionally, target cell killing with BiTE occurs in the absence of regular MHC peptide antigen recognition and costimulation and is therefore resistant to certain immune escape mechanisms affecting antigen presentation and those affecting generation of tumor-specific T cell clones. Because the CD3ε target of BiTE antibody construct is the same in CD8+ and CD4+ T cells of any phenotype, they are all engaged, leading to a polyclonal T cell activation, expansion and broad tumor cell killing.
Bispecific T-cell engagers offer immunotherapy in a manufacturing format which is both scalable and standardizable. In contrast to CAR T cells, T-cell engagers do not require initial lymphodepletion. Ex vivo manipulation of autologous cells has significant limitations, including the need for a centralized manufacturing infrastructure with extensively trained laboratory personnel to genetically modify each patient’s own T cells, use viral transduction which poses uncertain risks, are limited to the initial subset of T cells manipulated and infused, and still face uncertainty as to the optimal T cell phenotype to infuse.
Our Product Pipeline
APTN-101
We have recently reported the development of our first novel T cell engaging molecule, known as APTN-101, using our BRiTE technology. BRiTE focuses on the transport of difficult to deliver T cell targeting agents across the BBB allowing access to the immunoprivileged brain and overcoming the challenges with other immunotherapeutic approaches. This is accomplished by sequentially or simultaneously administering both BRiTE and specifically activated T cells by adoptive transfer. APTN-101 was designed to specifically redirect T cells against tumors expressing a well-characterized, mutated form of the EGFR, EGFRvIII, on a number of tumor types, including GBM. Because EGFRvIII is exclusively expressed on tumor cells, but not normal healthy cells, it represents an ideal target for immunotherapy.
APTN-101 (EGFRvIII x CD3 BRiTE) successfully activates human T cells against EGFRvIII expressing target cells, in the absence of any additional immunostimulatory signal, resulting in the secretion of Th-1-associated cytokines and tumor-cell killing. APTN-101 is similarly effective in vivo. Intravenous administration of APTN-101 induced consistent antitumor responses in mice bearing established, late-stage, aggressive, intracerebral patient-derived gliomas, rapidly achieving complete remission rates as high as 75% in the absence of apparent toxicity. Given the exquisite tumor-specificity of APTN-101, it represents a critical conceptual advance in safety contrary to target antigens having a promiscuous expression pattern.
The concept of BRiTE is the combination of novel T cell targeting agents with specifically activated polyclonal T cells. In order to exert antineoplastic affects against brain tumors, both the T cell targeting agent and T cells need to efficiently access areas that have long been considered as immunoprivileged. While circulating naïve T cells do not typically penetrate the CNS, activated T cells are known to cross the BBB to perform routine immunosurveillance of the central nervous system (Figure 1).
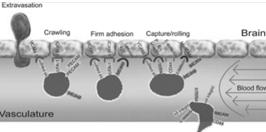
Figure 1- The process of T cells crossing the inflamed BBB is coordinating and sequential. Briefly, activated T cells initially arresting on the endothelium is mediated by the lymphocyte-associated antigen-1 (“LFA- 1”) and α4β1-integrin expressed on the T cells, respectively binding to the intracellular cell adhesion molecule 1 (“ICAM1”) and adhesion molecules vascular cell adhesion molecule 1 (“VCAM1”) on brain endothelial cells. Subsequently, the T cell crawling and polarization exclusively involve LFA-1 and ICAM1/2 interactions. After arriving at sites where are rich in the laminin isoform α4 but not laminin β5, the T cells use α6β1-integrin to traverse the endothelial basement membrane.
12
Upon intravenous administration, the T cell targeting agent (i.e., EGFRviii x CD3) binds to circulating T cells via its CD3 receptor and carries or “hitchhikes” the agent to tumors located behind the BBB. Studies in aggressive orthotopic GBM models have revealed that adoptive transfer of activated T cells significantly increases the biodistribution of intravenously administered EGFRvIII x CD3 BRiTE to orthotopic glioma (Figure 2).
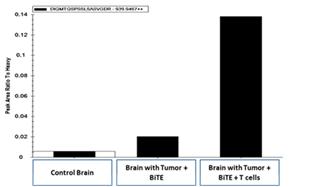
Figure 2- Mass spectroscopy demonstrates that pre-administration (four days) of ex vivo activated T cells increases the biodistribution of intravenously administered EGFRvIII x CD3 to the brain parenchyma.
BRiTE circumvents ordinary clonotypic T-cell specificity, potentially allowing any T-cell, regardless of endogenous specificity or phenotype, to exert an anti-neoplastic effect. In vivo experiments show that BRiTEs can reactivate potentially unresponsive, anergic T cells, such as those frequently encountered among TIL populations thereby enhancing the spread of T cells reactive towards other antigens (epitope spreading). Proximal contact between T cells and tumor cells could directly reactivate tumor infiltrating lymphocytes specific for cancer antigens other than directed by BRiTE, without cross-presentation. The EGFRvIII x CD3 BRiTE molecule has the potential to directly activate and expand pre-existing T cells among a polyclonal population that are specific for tumor antigens other than EGFRvIII. Indeed, others have discovered that by re-activating pre-existing T cell clones using a CD3 binding bispecific antibody specific for Wilms’ tumor protein (WT1) it is possible to induce effective and persistent epitope spreading responses to multiple antigens. If the clinical utility of this mechanism of epitope spreading is confirmed, this could provide an exciting mechanism to combat tumor heterogeneity.
Importantly, our data suggest that once APTN-101 reaches the brain, it can activate even suppressive Tregs to kill glioblastoma tumor cells by redirecting their natural granzyme-mediated cytotoxic potential and enhance a cytotoxic immune response. These findings not only highlight a new mechanism by which BRiTEs may circumvent certain aspects of Treg mediated suppression, but also have broader implications with regard to the natural functional role of activated, tumor infiltrating Tregs that ordinarily suppress and kill cytotoxic T lymphocytes in the tumor microenvironment.
By tethering cytotoxic effectors to target cells without the need for antigen presentation via the MHC, BRiTEs can furthermore overcome tumor immune escape mechanisms, such as the downregulation of MHC.
13
In addition to enhancing the biodistribution of EGFRvIII x CD3 BRiTE to the brain, the activated polyclonal T cells (compared to the addition of no activated polyclonal T cells or naïve polyclonal T cells) have the potential to restore effector T cells function at intracerebral sites (Figure 3) leading to cures in greater than 75% of animals.
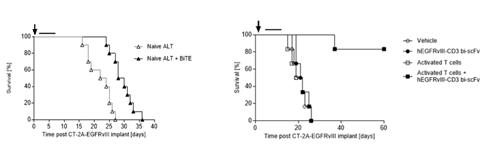
Figure 3- IV administration of activated T cells enhances hEGFRvIII-CD3 bi-scFv efficacy against syngeneic, highly-invasive, orthotopic glioma (right panel) compared to IV administration of naïve T cells when combined with hEGFRvIII-CD3 bi-scFv (left panel). The highly invasive murine glioma CT-2A-EGFRvIII was implanted orthotopically in human CD3 transgenic mice (females, 8-10 weeks old, n=10 per group).
Next, we hypothesized that the increase in efficacy observed with the pre-administration of activated polyclonal T cells would be abrogated if those T cells were to be blocked from entering the CNS parenchyma. Natalizumab, a clinically approved drug for the treatment of multiple sclerosis, functions by binding to polyclonal T cells and preventing their association with receptors involved in the process of extravasation. Remarkably, in cohorts of mice receiving adoptive transfer of activated polyclonal T cells along with treatment with the extravasation blocking molecule natalizumab, efficacy was decreased to levels observed in cohorts that did not receive adoptive transfer of activated polyclonal T cells (Figure 4).
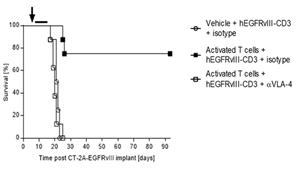
Figure 4- Blocking T cell extravasation with Natalizumab (αVLA-4) abrogates the observed increase in efficacy with adoptive cell transfer (n=8 per group). Natalizumab is a clinically approved drug for the treatment of multiple sclerosis that functions by blocking T cell extravasation.
We have also demonstrated an effective “antidote” for any potential toxicity that may result from administration of the EGFRvIII x CD3 BRiTE in a clinical setting. By administering a short peptide that spans the EGFRvIII mutation (PEPvIII), we have effectively blocked bispecific antibody function both in vitro and in vivo, providing a tool highly likely to aid in safe clinical administration of any EGFRvIII targeted bispecific antibody.
The above data demonstrates that BRiTE technology has the ability to transport difficult to deliver agents, including T cell targeting agents, across the BBB and demonstrate superior antineoplastic activity in aggressive orthotopic models of GBM while having an acceptable safety profile. This newly uncovered hitchhiking mechanism of drug delivery to the CNS provides an important tool to enhance the immunotherapy of brain tumors and has potentially far-reaching consequences for the treatment of other CNS disorders, such as Alzheimer’s or Parkinson’s disease, where issues regarding drug delivery to the CNS are relevant.
14
In summary, the results of preclinical studies demonstrate that the EGFRvIII targeting BRiTE may provide a safe, highly effective therapeutic option for GBM patients. Future studies will determine whether these results can be recapitulated in the clinical setting and whether BRiTEs favorably interact with other therapies that are currently employed as a standard-of-care for GBM patients.
APTN-101 Clinical Studies
The proposed Phase 1 study will evaluate a novel hEGFRvIII-CD3-biscFv Bispecific T cell engager (BRiTE) in patients diagnosed with pathologically documented supratentorial World Health Organization grade IV malignant glioma with an EGFR mutation (either newly diagnosed or at first progression/recurrence) at the Preston Robert Tisch Brain Tumor Center at Duke University.
The primary objective of this phase 1 study is to determine the safety and tolerability of BRiTE and recommend phase 2 dose of BRiTE injected with and without activated polyclonal T-cells in among a patient population that includes newly diagnosed patients after completion of standard of care therapy consisting of radiation and adjuvant temozolomide, and patients at first progression (defined as progression during or after standard of care radiation and adjuvant temozolomide).
Another secondary objective is to describe the PK in subjects treated with BRiTE. A population PK analysis will be performed to characterize the PK of BRiTE using the software Nonlinear Mixed Effects Modeling (NONMEM, version 7.2). Different structural PK models (e.g., 1 and 2 compartment) with linear or non-linear (e.g., Michaelis-Menten) kinetics will be fitted to the plasma concentration-time data of BRiTE.
Exploratory objectives include an evaluation of the pharmacodynamics effect of BRiTE, an evaluation of the formation and incidence of anti-BRiTE antibodies, and a description of overall survival and progression-free survival.
Our Intellectual Property
We strive to protect the proprietary technology that we believe is important to our business, including our product candidates and our processes. We seek patent protection in the United States and internationally for our product candidates, their methods of use and processes of manufacture, and any other technology to which we have rights, as appropriate. Additionally, we have licensed the rights to intellectual property related to certain of our product candidates, including patents and patent applications that cover the products or their methods of use or processes of manufacture. The terms of the licenses are described below under the heading “License Agreement.” We also rely on trade secrets that may be important to the development of our business.
We hold a world-wide exclusive license to three issued or allowed United States patents and one pending PTC patent application covering the enhanced delivery of drugs and other compounds to the brain and other tissues. The patents and patent applications that we licensed provide patent terms or anticipated patent terms ranging from 2031 to 2039 without patent term extensions.
Our success will in part depend on the ability to obtain and maintain patent and other proprietary rights in commercially important technology, inventions and know-how related to our business, the validity and enforceability of our patents, the continued confidentiality of our trade secrets, and our ability to operate without infringing the valid and enforceable patents and proprietary rights of third parties. We also rely on continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications we may own or license in the future, nor can we be sure that any of our existing patents or any patents we may own or license in the future will be useful in protecting our technology and products. For this and more comprehensive risks related to our intellectual property, please see “Risk Factors—Risks Related to Our Intellectual Property.”
15
License Agreement
Patent License Agreement with Duke University
Effective January 11, 2023, Private Adaptin entered into a patent license agreement (the “Duke License”) with Duke University, a nonprofit educational and research institution organized under the laws of North Carolina (“Duke University”), whereby Duke University granted us an exclusive license with a right to grant sublicenses to “BISPECIFIC EGFRvIII ANTIBODY ENGAGING MOLECULES,” “HUMAN BISPECIFIC EGFRvIII ANTIBODY AND CD3 ENGAGING MOLECULES,” “CERTAIN IMPROVED HUMAN BISPECIFIC EGFRvIII ANTIBODY ENGAGING MOLECULES” and “ENHANCED DELIVERY OF DRUGS AND OTHER COMPOUNDS TO THE BRAIN AND OTHER TISSUES” (together, the “precision medicine technology”). With this technology, which the Company assumed pursuant to the Merger, we intend to develop our BRiTE Platform, a combination of an immune cell engager with activated functional T-cells, which focuses on transporting difficult to deliver agents across the blood brain barrier, allowing access to the immunoprivileged central nervous system. Under the Duke License, we are required to use commercially reasonable efforts to obtain and retain the relevant governmental approvals and to commercialize the precision medicine technology. We must also use reasonable efforts to reach certain commercialization and research and development milestones as outlined in the Duke License.
On August 8, 2024, Private Adaptin entered into a Sponsored Research Agreement (the “SRA”), which the Company assumed pursuant to the Merger, whereby Duke University agreed to perform research exploring the administration methods of our BRiTE Platform for a fixed fee. The Duke License has been amended (the “First Amendment”) such that the Company has the option to add any invention conceived as a result of the performance under the SRA to the license.
As part of the consideration for the license, Private Adaptin issued Duke University shares, representing 5% of its issued and outstanding common stock on a fully diluted basis. Following the Merger, Duke University holds 2.0% of the combined Company. We also agreed to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities. These milestones include initiation of a Phase II or Phase III clinical trials, submission of applications for market approval in multiple jurisdictions including the United States, European Union and Japan and the initiation of post-approval commercial sales in the same jurisdictions. Based on an assumption that all milestones related to the current development program are met during the course of the Duke License, these milestone payments would total approximately $11.7 million. Under the terms of the Duke License, we must pay running royalties equal to low- to mid-single digit percentages of annual net sales, depending on the level of sales by us, our sublicensees and affiliates in that year, and subject to downward adjustment to low single digit percentages of our net annual sales in the event there is no valid claim of a patent for the product, with minimum annual royalty levels established. We also must pay Duke University low to mid-double-digit percentages of any sublicensing fees as set forth in the Duke License. We will be responsible for all patent expenses incurred by Duke University and must reimburse Duke University for previous patent expenses incurred by Duke University for filing and prosecution of the patent rights.
The term of the Duke License will extend until the expiration of the last to expire patent rights, subject to early termination as set forth in the Duke License. The foregoing descriptions of the Duke License, First Amendment and SRA do not purport to be complete and are qualified in their entirety by the terms and conditions of the Duke License, First Amendment and SRA, forms of which are attached hereto as Exhibits 10.1, 10.2 and 10.3, respectively, and are incorporated herein by reference.
Manufacturing and Supply
We contract with third parties for the manufacturing of all of our product candidates, and for pre-clinical and clinical studies and intend to continue to do so in the future. We do not own or operate any manufacturing facilities and we have no plans to build any owned clinical or commercial-scale manufacturing capabilities. We believe that the use of contract manufacturing organizations (“CMOs”) eliminates the need to directly invest in manufacturing facilities, equipment and additional staff. Although we rely on contract manufacturers, our personnel and consultants have extensive manufacturing experience overseeing CMOs.
16
We have produced the EGFRvIII x CD3 BRiTE in a fashion suitable for clinical translational and compatible with clinical biologic manufacturing infrastructure. This has included generating and certifying a Master Cell Bank and developing a scalable expression and tag-free purification and formulation process suitable for clinical translation. On the basis of our data and development work, we have had a CMO produce clinical-grade EGFRvIII x CD3 BRiTE suitable for clinical study.
As we further develop our product candidates, we expect to consider secondary or back-up manufacturers for both active pharmaceutical ingredient and drug product manufacturing. To date, our third-party manufacturers have met the manufacturing requirements for our product candidates in a timely manner. We expect third-party manufacturers to be capable of providing sufficient quantities of our product candidates to meet anticipated full-scale commercial demand but we have not assessed these capabilities beyond the supply of clinical materials to date. We currently engage CMOs on a “fee for services” basis based on our current development plans. We plan to identify CMOs and enter into longer-term contracts or commitments if and as we move our product candidates into Phase 3 clinical trials.
We believe alternate sources of manufacturing will be available to satisfy our clinical and potential future commercial requirements; however, we cannot guarantee that identifying and establishing alternative relationships with such sources will be successful, cost-effective, or completed on a timely basis without significant delay in the development or commercialization of our product candidates. All of the vendors we use are required to conduct their operations under current Good Manufacturing Practices (“cGMP”), a regulatory standard for the manufacture of pharmaceuticals.
Competition
The pharmaceutical industry is highly competitive and characterized by intense and rapidly changing competition to develop new technologies and proprietary products, particularly in some of the areas of high unmet medical need that we are targeting. Our potential competitors include both major and specialty pharmaceutical and biotechnology companies worldwide, many of which have far greater resources and access to capital than we do. In particular, Affimed N.V. and NexImmune, Inc. are studying the combination of immune cell engagers with T or NK cells using a different bispecific immune cell engager, and Amgen Inc. and Genentech, Inc. (a wholly-owned subsidiary of Roche Holding AG) have programs using a form of EGFRvIII x CD3 bispecific T cell engager (although neither are using them in combination with activated T cells). Our success will be based in part on our ability to identify, develop, and manage a portfolio of safe and effective product candidates that address the unmet needs of patients before our competitors.
Government Regulations
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of drugs, such as those we are developing. Along with our third-party contractors, we will be required to navigate the various preclinical, clinical, and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidates. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
FDA Regulation of Drugs
Before any of our drug product candidates may be marketed in the United States, they must be approved by the FDA. The process required by the FDA before drug product candidates may be marketed in the United States generally involves the following:
| ● | completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s current Good Laboratory Practices regulations; |
17
| ● | submission to the FDA of an IND, which must become effective before human clinical trials may begin and must be updated annually or when significant changes are made; |
| ● | approval by an independent institutional review board (“IRB”) or ethics committee for each clinical site before a clinical trial can begin; |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed product candidate for its intended purpose; |
| ● | preparation of and submission to the FDA of a New Drug Application (“NDA”) after completion of all required clinical trials; |
| ● | a determination by the FDA within 60 days of its receipt of an NDA to file the application for review; |
| ● | satisfactory completion of an FDA Advisory Committee review, if required by the FDA; |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with cGMP, and to assure that the facilities, methods, and controls are adequate to preserve the product’s continued safety, purity and potency, and of selected clinical investigational sites to assess compliance with current Good Clinical Practices; and |
| ● | FDA review and approval of the NDA to permit commercial marketing of the product for particular indications for use in the United States, which must be updated annually and when significant changes are made. |
The testing and approval processes require substantial time, effort, and financial resources and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all, and we may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our product candidates. The FDA may delay or refuse approval of an NDA if applicable regulatory criteria are not satisfied, or may require additional testing, information, and/or post-marketing testing and surveillance to monitor safety or efficacy of a product candidate.
If regulatory approval of a product candidate is granted, such approval may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy (“REMS”) plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development.
FDA Programs for Expedited Review and Increased Exclusivity
A sponsor may seek approval of a product candidate under Fast Track and Breakthrough Therapy programs designed to accelerate the FDA’s review and approval of new drug candidates that meet certain criteria, and/or to receive increased exclusivity under the orphan drug program. We intend to pursue these programs where our product candidates qualify.
Fast Track. A new drug candidate is eligible for Fast Track designation if it is intended to treat a serious or life-threatening condition, fill an unmet medical need, and demonstrate a significant improvement in the safety or effectiveness in the treatment of that condition.
A drug that receives Fast Track designation is eligible for the following:
| ● | more frequent meetings with FDA to discuss the drug’s development plan and ensure collection of appropriate data needed to support drug approval; |
18
| ● | more frequent written correspondence from FDA about the design of clinical trials; |
| ● | priority review to shorten the FDA review process for a new drug from ten months to six months; and |
| ● | rolling review, which means we can submit completed sections of its NDA for review by FDA, rather than waiting until every section of the application is completed before the entire application can be reviewed. |
Under the accelerated approval program, the FDA may approve an NDA on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Fast Track designation and priority review do not change the standards for approval but may expedite the development or approval process.
Breakthrough Therapy. A new drug candidate is eligible for Breakthrough Therapy designation if it is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on one or more clinically significant endpoints.
A drug that receives Breakthrough Therapy designation is eligible for the following:
| ● | All Fast Track designation features; |
| ● | Intensive guidance from the FDA on an efficient drug development program, beginning as early as Phase 1; and |
| ● | FDA organizational commitment involving senior managers. |
Orphan Drug Designation. Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug candidate intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that costs of research and development of the drug for the indication can be recovered by sales of the drug in the United States. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Although there may be some increased communication opportunities, orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a drug candidate that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same drug for the same indication for seven years, except in very limited circumstances, such as if the second applicant demonstrates the clinical superiority of its product or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
As in the United States, designation as an orphan drug for the treatment of a specific indication in the European Union must be made before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to ten years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan designated product.
The inability to obtain or failure to maintain adequate product exclusivity for our product candidates could have a material adverse effect on our business prospects, results of operations and financial condition.
19
Other Healthcare Laws and Compliance Requirements
Our sales, promotion, medical education, clinical research, and other activities following product approval will be subject to regulation by numerous regulatory and law enforcement authorities in the United States in addition to the FDA, including potentially the Federal Trade Commission, the Department of Justice, the Centers for Medicare and Medicaid Services (“CMS”), the U.S. Department of Health and Human Services (“DHHS”) Office of Inspector General, and other divisions of DHHS, and state and local governments.
Our business and our relationships with customers, physicians, and third-party payors are and will continue to be subject, directly and indirectly, to federal and state healthcare fraud and abuse laws and regulations. These laws also apply to the physicians and third-party payors who will play a primary role in the recommendation and prescription of our product candidates, if they become commercially available products. These laws may constrain the business or financial arrangements and relationships through which we might market, sell and distribute our products and will impact, among other things, any proposed sales, marketing and educational programs. There are also laws, regulations and requirements applicable to the award and performance of federal grants and contracts. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to them, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, disgorgement, the reimbursement of overpayments, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs, imprisonment, contractual damages, reputational harm, and diminished profits and earnings—any of which could adversely affect our ability to operate our business and our financial results.
Restrictions under applicable federal and state healthcare related laws and regulations include but are not limited to the following:
| ● | the federal Anti-Kickback Statute; |
| ● | the civil federal False Claims Act; |
| ● | the criminal federal False Claims Act; |
| ● | the Health Insurance Portability and Accountability Act, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and its implementing regulations (collectively, “HIPAA”); |
| ● | the civil monetary penalties statute; |
| ● | federal transparency laws, including the federal Physician Sunshine Act (“PSA”); and |
| ● | analogous or similar state, federal, and foreign laws, regulations, and requirements. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations involve substantial costs. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other laws, regulations, or other requirements that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, restitution exclusion from government funded healthcare programs, corporate integrity agreements, deferred prosecution agreements, debarment from government contracts and grants and refusal of future orders under existing contracts, contractual damages, the curtailment or restructuring of our operations and other consequences. If any of the physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, that person or entity may be subject to criminal, civil, or administrative sanctions, including exclusions from government funded healthcare programs. Moreover, availability of any federal grant funds which we may receive or for which we may apply is subject to federal appropriations law. Such grant funding may also be withdrawn or denied due to a violation of the above laws and/or for other reasons.
20
Coverage and Reimbursement; Healthcare Reform
Sales of pharmaceutical products depend significantly on the extent to which coverage and adequate reimbursement are provided by third-party payers. Third-party payers include state and federal government health care programs, managed care providers, private health insurers, and other organizations. Although we currently believe that third-party payers will provide coverage and reimbursement for our product candidates, if approved, we cannot be certain of this. Third-party payers are increasingly challenging the price, examining the cost-effectiveness and reducing reimbursement for medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. The United States government, state legislatures, and foreign governments have continued implementing healthcare reform and cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. We might need to conduct expensive clinical studies to demonstrate the comparative cost-effectiveness of our product candidates. Third-party payers might not consider the product candidates that we develop to be cost-effective and not cover or sufficiently reimburse for their use. It is time-consuming and expensive for us to seek coverage and reimbursement from third-party payers, as each payer will make its own determination as to whether to cover a product and at what level of reimbursement. Thus, one payer’s decision to provide coverage and adequate reimbursement for a product does not assure that another payer will provide coverage or that the reimbursement levels will be adequate. Moreover, a payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our product candidates to the extent we choose to develop or sell any product candidates outside of the United States. The approval process varies from country to country and the time may be longer or shorter than that required to obtain FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement, and privacy, can vary greatly from country to country.
Employees
As of the Effective Time of the Merger, we had four employees, all of whom are located in the United States. None of our employees is represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good.
Property
The Company owns no real property. We maintain a month-to-month membership providing mail services and shared space for meetings and other business activities at 3540, Toringdon Way, Suite 200, #250, Charlotte, North Carolina. The current rent is approximately $100 per month.
Litigation
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
Available Information
Our principal offices are located at 3540 Toringdon Way, Suite 200, #250, Charlotte, NC 28277 and our telephone number is (888) 609-1498. Our website is www.adaptinbio.com, and we can be contacted at info@adaptinbio.com. We are subject to the informational requirements of the Exchange Act and file or furnish reports, proxy statements, and other information with the SEC. Such reports and other information filed by us with the SEC will be available free of charge on our website at www.adaptinbio.com when such reports are available on the SEC’s website. The SEC maintains a website that contains reports, proxy and information statements, and other information that issuers file electronically with the SEC at www.sec.gov.
The contents of the websites referred to above are not incorporated into this filing. Further, our references to the URLs for these websites are intended to be inactive textual references only.
21
RISK FACTORS
This Report contains forward-looking statements which involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those set forth in the following risk factors.
Risk Factors Summary
Risks Related to Our Business and Operations
| ● | We have generated no revenue from commercial sales to date and our future profitability is uncertain. |
| ● | The report of our independent registered accounting firm expresses substantial doubt about our ability to continue as a going concern. |
| ● | We have limited access to the capital markets and even if we can raise additional funding, we may be required to do so on terms that are dilutive to our stockholders. |
| ● | If we fail to obtain the additional capital necessary to fund our operations, we will be unable to continue or complete our product development and our business will be substantially harmed. |
| ● | We may expand our business through the acquisition of rights to new drug candidates that could disrupt our business, harm our financial condition and may also dilute our stockholders’ ownership. |
| ● | If any future collaborations with third parties for the discovery, development and commercialization of our product candidates fail to lead to commercial products, and we may never receive milestone payments or future royalties under these agreements. |
| ● | The marketing approval process of the FDA is lengthy, time consuming and inherently unpredictable, and if we were ultimately unable to obtain marketing approval for the product candidates we intend to develop, our business will be substantially harmed. |
| ● | Our product candidates are in the early stages of development. |
| ● | We have relied and may in the future rely on third parties to conduct investigator-sponsored trials of our products, which is cost-effective but affords the investigators the ability to retain significant control over the design and conduct of the trials, and use of the data generated from their efforts. |
| ● | We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our products. |
| ● | Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities. |
| ● | Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. |
| ● | We currently rely significantly on third parties to conduct our nonclinical testing and clinical studies and other aspects of our development programs. If those third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products. |
| ● | The results of our current or future clinical trials may not support our product candidate claims or may result in the discovery of unexpected adverse side effects. |
| ● | Even if approved, our product candidates and future products may never achieve market acceptance. |
| ● | Our revenue stream will depend upon third-party reimbursement. |
| ● | If a product candidate is approved and we are unable to establish satisfactory sales and marketing capabilities or secure a sales and marketing partner, we may not successfully commercialize our approved product candidates. |
| ● | We will rely on the availability of specific off-the-shelf components. |
| ● | If we market any of our products in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties. |
| ● | Our products will face significant competition in the markets for such products, and if they are unable to compete successfully, our business will suffer. |
| ● | After approval, if obtained, our products will remain subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional risk and expense, including the FDA withdrawing or modifying approval for our products and product recalls that could harm our reputation, business and financial results. |
22
| ● | We may be adversely affected by a failure or compromise from a cyberattack or data breach, which could have an adverse effect on our business. |
| ● | We may undertake international operations, which will subject us to risks inherent with operations outside of the United States. |
| ● | We may not be successful in hiring and retaining key employees. |
| ● | Managing our growth as we expand operations may strain our resources. |
Risks Related to Our Intellectual Property
| ● | We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our current and future licensors. |
| ● | Our ability to protect and enforce any patents we may obtain does not guaranty that we will secure the right to commercialize such patents. |
| ● | If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish, and our business and competitive position would suffer. |
| ● | We rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties, our trade secrets may become known to our competitors. |
| ● | If we or our third-party suppliers are claimed to or found to be infringing on patents or trade secrets owned by others, we may be forced to cease or alter our product development efforts, obtain a license to continue the development or sale of our products, and/or pay damages. |
Risks Related to Ownership of our Common Stock
| ● | If we are unable to register in a timely manner the shares of Common Stock issued to stockholders in the Merger or the Offering, we may be subject to certain liquidated damages. |
| ● | There is currently no market for our Common Stock and there can be no assurance that any market will ever develop and our Common Stock may not be eligible for listing or quotation on any securities exchange or over-the-counter trading system. |
| ● | The market price and trading volume of our Common Stock may be volatile and could decline significantly. |
| ● | The designation of our Common Stock as “penny stock” or FINRA sales practice requirements may limit the liquidity of our Common Stock. |
| ● | Because we do not intend to pay dividends, stockholders will benefit from an investment in our Common Stock only if it appreciates in value. |
| ● | Because we became a reporting company under the Exchange Act by means other than a traditional underwritten initial public offering, we may not be able to attract the attention of research analysts at major brokerage firms. |
| ● | Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management. |
| ● | The issuance of our preferred stock could adversely affect the holders of our Common Stock in some circumstances. |
| ● | We are subject to the reporting requirements of federal securities laws, which can be expensive and may divert resources from other projects, thus impairing our ability to grow. |
| ● | Our principal stockholders will have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company. |
23
Risks Related to Our Business and Operations
We have generated no revenue from commercial sales to date and our future profitability is uncertain.
Private Adaptin was incorporated as Centaur Bio Inc., a Delaware corporation in 2021. The combined Company has a limited operating history and our business is subject to all of the risks inherent in the establishment of a new business enterprise, which make our prospects hard to evaluate. Any evaluation of our business and our prospects must be considered in light of the uncertainties, problems, expenses, difficulties, complications and delays frequently encountered in connection with development and expansion of a new business enterprise. Since inception, we have incurred losses and expect to continue to operate at a net loss for at least the next several years as we commence our research and development efforts, conduct clinical trials and develop manufacturing, sales, marketing and distribution capabilities. There can be no assurance that the products under development by us will be approved for sale in the United States or elsewhere. Furthermore, there can be no assurance that if such products are approved, they will be successfully commercialized, and the extent of our future losses and the timing of our profitability are highly uncertain. Many of these factors are beyond the control of our management. If we are unable to achieve profitability, we may be unable to continue our operations.
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern.
Our auditor, KNAV CPA LLP, has indicated in their report on our financial statements for the fiscal year ended December 31, 2023, that conditions exist that raise substantial doubt about our ability to continue as a going concern due to our recurring losses from operations and significant accumulated deficit. In addition, we continue to experience negative cash flows from operations. A “going concern” opinion could impair our ability to finance our operations through the sale of equity. Our ability to continue as a going concern will depend upon the availability of equity financing which represents the primary source of cash flows that will permit us to meet our financial obligations as they come due and continue our research and development efforts.
We have limited access to the capital markets and even if we can raise additional funding, we may be required to do so on terms that are dilutive to our stockholders.
We have limited access to the capital markets to raise capital. The capital markets have been unpredictable in the recent past for unprofitable companies such as ours. In addition, it is generally difficult for development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able to raise often depends on variables in market conditions that are beyond our control. As a result, we may not be able to secure financing on terms attractive to us, or at all. If we are able to consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition and our continued viability will be materially adversely affected. If we are able to secure future financing, it may be done on terms that are potentially dilutive to our stockholders.
If we fail to obtain the additional capital necessary to fund our operations, we will be unable to continue or complete our product development and our business will be substantially harmed.
The net proceeds from our Offering are not sufficient to capitalize the development and commercialization of the product candidates we intend to develop and we will need to continue to seek capital from time to time to continue development of the BRiTE platform beyond the initial Phase 1 clinical trials and to acquire and develop other product candidates. Our first product is not expected to be commercialized until at least 2028, if at all, and we cannot provide any assurances that any revenues it may generate in the future will be sufficient to fund our ongoing operations.
We believe the Company can fund its operations through the planned Phase 1 clinical trial and into 2026 with net proceeds from the Offering, if we raise the Maximum Offering amount of approximately $8.5 million. As of the date of this Report, we have raised approximately $4.8 million in the Offering. Accordingly, we believe that we will need to raise substantial additional capital to fund our continuing operations and the development and commercialization of our product candidates in or before 2026. Our business or operations may change in a manner that would consume available funds more rapidly than anticipated and substantial additional funding may be required to maintain operations, fund expansion, develop new or enhanced products, acquire complementary products, business or technologies or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a change in the global healthcare environment. In addition, if our product candidates are commercialized, we may need to accelerate the growth of our sales capabilities and distribution beyond what is currently envisioned, and this would require additional capital. However, we may not be able to secure funding when we need it or on acceptable terms. We may not be able to raise sufficient funds to commercialize the product candidates we intend to develop.
24
If we cannot raise adequate funds to satisfy our capital requirements, we will have to delay, scale back or eliminate our research and development activities, clinical studies or future operations. We may also be required to obtain funds through arrangements with collaborators, which arrangements may require us to relinquish rights to certain products that we otherwise would not consider relinquishing, including rights to future product candidates or certain major geographic markets. This could result in sharing revenues that we might otherwise retain for ourselves. Any of these actions may harm our business, financial condition and results of operations. If adequate funds cannot be secured to sustain our business operations, our board of directors may decide that it is in the best interest of our stockholders to dissolve our Company and liquidate our assets.
The amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs; the progress, timing and scope of our preclinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals; the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to the development and commercialization of our products.
We may expand our business through the acquisition of rights to new drug candidates that could disrupt our business, harm our financial condition and may also dilute current stockholders’ ownership interests in us.
Our business strategy includes expanding our products and capabilities, and we may seek acquisitions of drug candidates or technologies to do so. Acquisitions involve numerous risks, including substantial cash expenditures; potentially dilutive issuance of equity securities; incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; difficulties in assimilating the acquired technologies or the operations of the acquired companies; diverting our management’s attention away from other business concerns; risks of entering markets in which we have limited or no direct experience; and the potential loss of our key employees or key employees of the acquired companies.
We cannot assure you that any acquisition will result in short-term or long-term benefits to us. We may misjudge the value or worth of an acquired product, company or business. In addition, our future success would depend in part on our ability to manage the rapid growth associated with acquisitions. We cannot assure you that we will be able to make the combination of our business with that of acquired products, businesses or companies work or be successful. Acquisitions and the subsequent integration of new assets, businesses, key personnel, customers, vendors, and suppliers require significant attention from our management and could result in a diversion of resources from our existing business, which in turn could have an adverse effect on our operations. Furthermore, the development or expansion of our business or any acquired products, business or companies may require a substantial capital investment by us. We may not have these necessary funds, or they might not be available to us on acceptable terms or at all. We may also seek to raise funds by selling shares of our preferred or Common Stock, which could dilute each current stockholder’s ownership interest in us.
We may, in the future, seek to enter into collaborations with third parties for the discovery, development and commercialization of our product candidates. If our collaborators cease development efforts under our collaboration agreements, or if any of those agreements are terminated, these collaborations may fail to lead to commercial products, and we may never receive milestone payments or future royalties under these agreements.
We may in the future seek to enter into agreements with other third-party collaborators for research, development and commercialization of other therapeutic technologies or product candidates. Biopharmaceutical companies are our likely future collaborators for any marketing, distribution, development, licensing or broader collaboration arrangements. If we fail to enter into future collaborations on commercially reasonable terms, or at all, or such collaborations are not successful, we may not be able to execute our strategy to develop our product candidates or therapies that we believe could benefit from the resources of either larger biopharmaceutical companies or those specialized in a particular area of relevance.
25
With our existing Duke License and with any future collaboration agreements, we have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Moreover, our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. Collaborations involving our product candidates pose the following risks to us:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ● | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on preclinical studies or clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| ● | collaborators with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to litigation or potential liability; |
| ● | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| ● | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
As a result of the foregoing, our current and any future collaboration agreements may not lead to development or commercialization of our product candidates in the most efficient manner or at all. If a collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated. Any failure to successfully develop or commercialize our product candidates pursuant to our current or any future collaboration agreements could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Moreover, to the extent that any of our existing or future collaborators were to terminate a collaboration agreement, we may be forced to independently develop these product candidates, including funding preclinical studies or clinical trials, assuming marketing and distribution costs and defending intellectual property rights, or, in certain instances, abandon product candidates altogether, any of which could result in a change to our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
26
The marketing approval process of the FDA is lengthy, time consuming and inherently unpredictable, and if we were ultimately unable to obtain marketing approval for the product candidates we intend to develop, our business will be substantially harmed.
We cannot commercialize our product candidates in the United States without first obtaining approval from the FDA to market each product candidate. None of the product candidates we intend to develop have gained marketing approval in the United States and we cannot guarantee that we will gain the required marketing approval. Our business is substantially dependent on our ability to complete the development of, obtain marketing approval for, and successfully commercialize our product candidates in a timely manner. Our product candidates could fail to receive marketing approval for many reasons, including among others:
| ● | The FDA may disagree with the design or implementation of our clinical trials; |
| ● | Our clinical trials may not produce results demonstrating that our products are safe and effective; and |
| ● | The FDA could determine that our manufacturing processes and procedures are not sufficient to support approval. |
In addition, the process of seeking regulatory clearance or approval to market the product candidates we intend to develop is expensive and time consuming and, notwithstanding the effort and expense incurred, clearance or approval is never guaranteed. If we are not successful in obtaining timely clearance or approval of our products from the FDA, we may never be able to generate any revenue and may be forced to cease operations. The NDA process is costly, lengthy and uncertain. Any NDA application filed by the Company will have to be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the product for its intended use.
Obtaining clearances or approvals from the FDA and from the regulatory agencies in other countries could result in unexpected and significant costs for us and consume management’s time and other resources. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development. The FDA and other agencies could ask us to supplement our submissions, collect non-clinical data, conduct additional clinical trials or engage in other time-consuming actions, or simply deny our applications. In addition, even if we obtain an NDA approval or pre-market approvals in other countries, the approval could be revoked or other restrictions imposed if post-marketing studies and surveillance demonstrates safety issues or lack of effectiveness after commercialization. We cannot predict with certainty how, or when, the FDA will act. If we are unable to obtain the necessary regulatory approvals, our financial condition and cash flow may be adversely affected, and our ability to grow domestically and internationally may be limited. Additionally, even if cleared or approved, the Company’s products may not be approved for the specific indications that are most necessary or desirable for successful commercialization or profitability.
Our product candidates are in the early stages of development.
Extensive further laboratory and specific clinical testing will be required prior to regulatory approval of any of our product candidates. Adverse or inconclusive results from preclinical testing or clinical trials of our product candidates may substantially delay, or halt entirely, any further development of one or more of our products.
We have relied and may in the future rely on third parties to conduct investigator-sponsored trials (“ISTs”) of our products, which is cost-effective for us but affords the investigators the ability to retain significant control over the design and conduct of the trials, as well as the use of the data generated from their efforts.
We have relied and may in the future rely on third parties to conduct and sponsor clinical trials. Such ISTs may provide us with valuable clinical data that can inform our future development strategy in a cost-efficient manner, but we do not control the design or conduct of the ISTs, and it is possible that the FDA or non-United States regulatory authorities will not view these ISTs as providing adequate support for future clinical trials, whether controlled by us or third parties, for any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results.
27
These arrangements provide us limited information rights with respect to the ISTs, including access to and the ability to use and reference the data, including for our own regulatory filings, resulting from the ISTs. However, we would not have control over the timing and reporting of the data from ISTs, nor would we own the data from the ISTs. If we are unable to confirm or replicate the results from the ISTs or if negative results are obtained, we would likely be further delayed or prevented from advancing further clinical development. Further, if investigators or institutions breach their obligations with respect to the clinical development of our product candidates, or if the data proves to be inadequate compared to the first-hand knowledge we might have gained had the ISTs been sponsored and conducted by us, then our ability to design and conduct any future clinical trials ourselves may be adversely affected.
Clinical trials necessary to support NDA approval of our product candidates will be time-consuming and expensive. Delays or failures in our clinical trials will prevent us from commercializing our products and will adversely affect our business, operating results and prospects and could cause us to cease operations.
Initiating and completing clinical trials necessary to support NDA approval of our product candidates and other new products will be time consuming and expensive, and the outcome uncertain. Moreover, the results of early clinical trials are not necessarily predictive of future results, and any product we advance into clinical trials may not have favorable results in later clinical trials.
Each modification to the protocol during a clinical trial has to be submitted to the FDA. This could result in the delay or halt of a clinical trial while the modification is evaluated. In addition, depending on the quantity and nature of the changes made, the FDA could take the position that the data generated by the clinical trial is not poolable because the same protocol was not used throughout the trial. This might require the enrollment of additional subjects, which could result in the extension of the clinical trial and the FDA delaying clearance or approval of a product. Any such delay could have a material adverse effect on our business and results of operations. Serious injury or death resulting from a failure of one of our drug candidates during current or future clinical trials could also result in the FDA delaying our clinical trials or denying or delaying clearance or approval of a product.
Even though an adverse event may not be the result of the failure of our drug candidate, the FDA or an IRB could delay or halt a clinical trial for an indefinite period of time while an adverse event is reviewed, and likely would do so in the event of multiple such events.
Any delay or termination of our current or future clinical trials as a result of the risks summarized above, including delays in obtaining or maintaining required approvals from IRBs, delays in patient enrollment, the failure of patients to continue to participate in a clinical trial, and delays or termination of clinical trials as a result of protocol modifications or adverse events during the trials, may cause an increase in costs and delays in the filing of any product submissions with the FDA, delay the approval and commercialization of our products or result in the failure of the clinical trial, which could adversely affect our business, operating results and prospects.
Modifications to our products may require new NDA approvals.
Once a particular product receives FDA approval or clearance, expanded uses or uses in new indications of our products may require additional human clinical trials and new regulatory approvals or clearances, including additional IND and NDA submissions and premarket approvals before we can begin clinical development, and/or prior to marketing and sales. If the FDA requires new clearances or approvals for a particular use or indication, we may be required to conduct additional clinical studies, which would require additional expenditures and harm our operating results. If the products are already being used for these new indications, we may also be subject to significant enforcement actions. Conducting clinical trials and obtaining clearances and approvals can be a time-consuming process, and delays in obtaining required future clearances or approvals could adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
28
We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our products.
We are, and will for the foreseeable future continue to be, wholly dependent on third party CMOs for the timely supply of adequate quantities of our products which meet or exceed requisite quality and production standards for use in clinical and nonclinical studies. Given the extensive risks, scope, complexity, cost, regulatory requirements and commitment of resources associated with developing the capabilities to manufacture one or more of our products, we have no present plan or intention of developing in-house manufacturing capabilities for nonclinical, clinical or commercial scale production, beyond our current supervision and management of our third-party contract manufacturers. In addition, in order to balance risk and conserve financial and human resources, we have and may continue from time to time to defer commitment to production of product, which could result in delays to the continued progress of our clinical and nonclinical testing.
Currently, we do not have alternative CMOs to back up our primary CMOs. Identification of and discussions with other CMOs may be protracted and/or unsuccessful, or these new CMOs may be unsuccessful in producing the same results as the current primary CMOs producing the material. Therefore, if our primary CMOs become unable or unwilling to perform their required activities, we could experience protracted delays or interruptions in the supply of clinical trial material and, ultimately, product for commercial sale, which would materially and adversely affect our development programs, commercial activities, operating results and financial condition. In addition, the FDA or regulatory authorities outside of the United States may require us to have an alternate manufacturer before approving any product candidate for marketing and sale in the United States or abroad and securing such alternate manufacturer, if possible, could result in considerable additional time and cost prior to approval.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling-up initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product candidate and quality assurance testing and shortages of qualified personnel. Our product candidates have not been manufactured at the scale we believe will be necessary to maximize their commercial value, and accordingly, we may encounter difficulties in attempting to scale-up production and may not succeed in that effort on a timely basis or at all. In addition to the foregoing, the process of manufacturing our products is complex, highly regulated and subject to several risks, including but not limited to the following:
| ● | We, and our CMOs, must comply with the FDA’s cGMP, regulations and guidance. We, and our CMOs, may encounter difficulties in achieving quality control and quality assurance and may experience shortages in qualified personnel. We, and our CMOs, are subject to inspections by the FDA and comparable agencies in other jurisdictions to confirm compliance with applicable regulatory requirements. Any failure to follow cGMP or other regulatory requirements or any delay, interruption or other issues that arise in the manufacture, fill-finish, labeling, packaging, or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to comply with regulatory requirements, or a failure to pass any regulatory authority inspection, could significantly impair our ability to develop and commercialize our products, including leading to significant delays in the availability of products for our clinical studies or the termination or hold on a clinical study, or the delay or prevention of a filing or approval of marketing applications for our product candidates. Significant noncompliance could also result in the imposition of sanctions, including injunctions, civil penalties, failure of regulatory authorities to grant marketing approvals for our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions, adverse publicity, and criminal prosecutions, any of which could damage our reputation. If we are not able to maintain regulatory compliance, we may not be permitted to market our products and/or may be subject to product recalls, seizures, injunctions, or criminal prosecution. Any adverse developments affecting manufacturing operations for our products or our CMOs generally, may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our products. Once our product candidates are approved, we may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. |
| ● | The manufacturing facilities in which our products are made could be adversely affected by equipment failures, plant closures, capacity constraints, competing customer priorities or changes in corporate strategy or priorities, process changes or failures, changes in business models or operations, materials or labor shortages, natural disasters, power failures and numerous other factors. |
29
| ● | We are wholly dependent upon CMOs for the timely supply of adequate quantities of requisite quality product for our nonclinical, clinical and, if approved by regulatory authorities, commercial scale production. |
| ● | The process of manufacturing biologics is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or in the manufacturing facilities in which our products are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. |
We rely completely on third parties, most of which are sole source suppliers, to supply drug substance and manufacture drug product for our clinical trials and preclinical studies and intend to rely on other third parties to produce commercial supplies of product candidates, and our dependence on third parties could adversely impact our business.
We are completely dependent on third-party suppliers, most of which are sole source suppliers of the drug substance and drug product for our product candidates. We regularly evaluate potential alternate sources of supply but there can be no assurance that any such suppliers would be available, acceptable or successful. The costs of manufacturing our drug candidates are high, and we will require additional capital to ensure that we can maintain an adequate supply to conduct our contemplated development programs.
If our third-party suppliers do not supply sufficient quantities for product candidates to us on a timely basis and in accordance with applicable specifications and other regulatory requirements, there could be a significant interruption of our supplies, which would adversely affect clinical development of the product candidate, including affecting our ability to enroll in and timely progress clinical trials. Furthermore, if any of our CMOs cannot successfully manufacture material that conforms to our specifications and with regulatory requirements, we will not be able to secure and/or maintain regulatory approval for our product candidates.
We will also rely on our CMOs to purchase from third-party suppliers the materials necessary to produce our product candidates for our anticipated clinical trials. There are a small number of suppliers for certain capital equipment and raw materials used to manufacture our product candidates. We do not have any control over the process or timing of the acquisition of these raw materials by our contract manufacturers. Moreover, we currently do not have agreements in place for the commercial production of these raw materials. Any significant delay in the supply of a product candidate or the raw material components thereof for an ongoing clinical trial could considerably delay completion of that clinical trial, product candidate testing, and potential regulatory approval of that product candidate.
We do not expect to have the resources or capacity to commercially manufacture any of our proposed product candidates if approved and will likely continue to be dependent on CMOs. Our dependence on third parties to manufacture and supply us with clinical trial materials and any approved product candidates may adversely affect our ability to develop and commercialize our product candidates on a timely basis.
Governments may impose price controls, which may adversely affect our future profitability.
We intend to seek approval to market our future product candidates in the United States and potentially in foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to our product candidates. In some foreign countries, particularly in the European Union, the pricing of prescription pharmaceuticals and biologics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. If reimbursement of our future products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability. We face potential product liability exposure and, if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
30
The use of our product candidates in clinical trials and the sale of any product candidates for which we may obtain marketing approval expose us to the risk of product liability claims. Product liability claims may be brought against us or any future development partners by participants enrolled in our clinical trials, patients, health care providers, or others using, administering, or selling our product candidates. If we cannot successfully defend ourselves against any such claims, or have insufficient insurance protection, we would incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
| ● | withdrawal of clinical trial participants; |
| ● | termination of clinical trial sites or entire trial programs; |
| ● | costs of related litigation; |
| ● | substantial monetary awards to trial participants or other claimants; |
| ● | decreased demand for our product candidates and loss of revenue; |
| ● | impairment of our business reputation; |
| ● | diversion of management and scientific resources from our business operations; and |
| ● | the inability to commercialize our product candidates. |
In the future, we anticipate that we will need to obtain additional or increased product liability insurance coverage and we are uncertain whether such increased or additional insurance coverage can be obtained on commercially reasonable terms, if at all.
We have obtained limited product liability insurance coverage for our clinical trials domestically and in selected foreign countries where we are conducting clinical trials. As such, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and in the future we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. We intend to expand our insurance coverage for product candidates to include the sale of commercial products if we obtain marketing approval for our product candidates in development; however, we may be unable to obtain commercially reasonable product liability insurance for any product candidates approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our working capital and adversely affect our business.
Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for the some of the categories of risk that our business may encounter, although we do intend to obtain customary insurance coverage. No assurance can be given that we will be able to obtain and/or maintain insurance with adequate levels of coverage. Any significant, uninsured liability may require us to pay substantial amounts, which would adversely affect our working capital and results of operations.
Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit.
We may not be able to commence or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population; the nature of the trial protocol; the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; the availability of appropriate clinical trial investigators; support staff; and proximity of patients to clinical sites and ability to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products that treat the same indications.
31
We currently rely significantly on third parties to conduct our nonclinical testing and clinical studies and other aspects of our development programs. If those third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products.
We do not have the ability to independently conduct our pre-clinical and clinical trials for our products and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories and others to assist in the design and conduct of non-clinical and clinical studies of our product candidates, with interpretation of the results of those studies and with regulatory activities and expect to continue to outsource all or a significant amount of such activities. As a result, many important aspects of our development programs are and will continue to be outside our direct control and our third parties may not perform their activities as required or expected, including the maintenance of Good Laboratory Practices or Good Clinical Practices compliance, which are ultimately our responsibility to ensure. Further, such third parties may not be as committed to the success of our programs as our own employees and, therefore, may not devote the same time, thoughtfulness or creativity to completing projects or problem-solving as our own employees would. To the extent we are unable to successfully manage the performance of our third parties, our business may be adversely affected. If these third parties do not successfully carry out their contractual duties or regulatory obligations, meet expected deadlines or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, our products on a timely basis, if at all. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control. The occurrence of any of the foregoing may adversely affect our business, operating results and prospects.
The results of our current or future clinical trials may not support our product candidate claims or may result in the discovery of unexpected adverse side effects.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our drug candidate claims or that the FDA or foreign authorities will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our drug candidates are safe and effective for the proposed indicated uses. If the FDA concludes that the clinical trials for our product candidates, or any other product for which we might seek clearance, has failed to demonstrate safety and effectiveness, we will not receive FDA clearance to market that product in the United States for the indications sought.
In addition, such an outcome could cause us to abandon the product candidate and might delay development of others. Any delay or termination of our clinical trials will delay the filing of any product submissions with the FDA and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile. In addition, our clinical trials for our product candidates may involve a relatively small patient population in some cases. Because of the small sample size, our results may not be indicative of future results.
Even if approved, our product candidates and future products may never achieve market acceptance.
Even if approved, our product candidates and future products that we may develop may never gain market acceptance among physicians, patients, third-party payers and the medical community. The degree of market acceptance of any of our products will depend on a number of factors, including the actual and perceived effectiveness and reliability of our products; the results of any long-term clinical trials relating to use of our products; our ability to convert patients from our clinical trials into users of our commercial products, once approved; the availability, relative cost and perceived advantages and disadvantages of alternative technologies; the degree to which treatments using our products are approved for reimbursement by public and private insurers; the willingness of patients to pay out of pocket in the absence of government or third-party coverage; the strength of our marketing and distribution infrastructure; the level of education and awareness among physicians and hospitals concerning our products; and prevalence and severity of any side effects. In addition, our efforts to educate the medical community and third-party payers regarding the benefits of our products may require significant resources and may never be successful. If approved, the failure of our product candidates resulting from the BRiTE platform or any of our other products to significantly penetrate current or new markets would negatively impact our business, financial condition and results of operations.
32
To be commercially successful, physicians must be persuaded that using our products for treatment of cancer, infectious diseases and other diseases are effective alternatives to existing therapies and treatments.
We believe that physicians will not widely adopt our products unless they determine, based on experience, clinical data, and published peer-reviewed journal articles, that the use of our products provides an effective alternative to other means of treating our target indications or any other disease that our products are approved to treat. Patient studies or clinical experience may indicate that treatment with our products does not provide patients with sufficient benefits in quality of life. We believe that recommendations and support for the use of our products from influential physicians will be essential for widespread market acceptance. Our products are still in the preclinical development stage and it is premature to attempt to gain support from physicians at this time. We can provide no assurance that such support will ever be obtained. If our products do not receive such support from these physicians and from long-term data, physicians may not use or continue to use, and hospitals may not purchase or continue to purchase, our products.
Even if our products are approved by regulatory authorities, if we, our CMOs or our suppliers fail to comply with ongoing FDA regulation or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain clearance or approval, and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA. In particular, we, our CMOs and our suppliers are required to comply with the FDA’s cGMP regulations for the manufacture of our products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. Regulatory bodies, such as the FDA, enforce these regulations through periodic inspections. The failure by us, our CMOs or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, enforcement actions by the FDA.
If any of these actions were to occur it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, such clearance or approval may be subject to limitations on the intended uses for which the product may be marketed and reduce the potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that the product promotional materials, labeling, training or other marketing or educational activities constitute promotion of an unapproved use, it could request that we or our commercialization partners cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider such training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with adverse event and pharmacovigilance reporting requirements, including the reporting of adverse events which occur in connection with, and whether or not directly related to, our products. For example, the FDA may approve the NDA with a REMS plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to recall, replace or refund the cost of any product we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
33
Our revenue stream will depend upon third-party reimbursement.
The commercial success of our products in both domestic and international markets will be substantially dependent on whether third-party coverage and reimbursement is available for patients that use our products. However, the availability of insurance coverage and reimbursement for newly approved therapies is uncertain, and therefore, third-party coverage may be particularly difficult to obtain even if our products are approved by the FDA as safe and efficacious. Patients using existing approved therapies are generally reimbursed all or part of the product cost by Medicare or other third-party payors. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly challenging the prices and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy, and increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs, and, as a result, they may not cover or provide adequate payment for these products.
Significant uncertainty exists as to the reimbursement status for newly approved drug products, including coding, coverage and payment. There is no uniform policy requirement for coverage and reimbursement for drug products among third-party payers in the United States; therefore coverage and reimbursement for drug products can differ significantly from payer to payer. The coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payer separately, with no assurance that coverage and adequate payment will be applied consistently or obtained. The process for determining whether a payer will cover and how much it will reimburse a product may be separate from the process of seeking approval of the product or for setting the price of the product. Even if reimbursement is provided, market acceptance of our products may be adversely affected if the amount of payment for our products proves to be unprofitable for healthcare providers or less profitable than alternative treatments or if administrative burdens make our products less desirable to use. Third-party payer reimbursement to providers of our products, if approved, may be subject to a bundled payment that also includes the procedure of administering our products or third-party payers may require providers to perform additional patient testing to justify the use of our products. To the extent there is no separate payment for our products, there may be further uncertainty as to the adequacy of reimbursement amounts.
The containment of healthcare costs is a priority of federal, state and foreign governments and the prices of drug products have been a focus in this effort. The continuing efforts of government, private insurance companies and other organizations to contain or reduce costs of healthcare may adversely affect our ability to set as high a price for our products as we might otherwise and the rate and scope of adoption of our products by healthcare providers. We expect that federal, state and local governments in the United States, as well as governments in other countries, will continue to consider legislation directed at lowering the total cost of healthcare. In addition, in certain foreign markets, the pricing of drug products is subject to government control and reimbursement may in some cases be unavailable or insufficient. It is uncertain whether and how future legislation, whether domestic or abroad, could affect prospects for our product candidates or what actions governmental or private payers for healthcare treatment and services may take in response to any such healthcare reform proposals or legislation. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, may prevent or limit our ability to generate revenue, attain profitability or commercialize our product candidates.
These potential courses of action are unpredictable and the potential impact of new legislation on our operations and financial position is uncertain, but may result in more rigorous coverage criteria, lower reimbursement and additional downward pressure on the price we may receive for an approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products, if approved.
34
We currently have no sales and marketing organization. If a product candidate is approved and we are unable to establish satisfactory sales and marketing capabilities and/or secure a sales and marketing partner, we may not successfully commercialize our approved product candidates.
We do not have experience in selling or marketing. To commercialize our products, if approved, in the United States and other jurisdictions in which we may seek approvals, we must build our marketing, sales, managerial and other non-technical capabilities or make arrangements with third parties to perform these services and we may not be successful in doing so. Despite the experience of individual members of management, we have limited experience as a company in the marketing and sale of pharmaceutical products and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team.
In addition, we may not be able to enter into collaboration agreements with sales and marketing partners on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure, we may not realize a positive return on this investment. In addition, we will have to compete with established and well-funded pharmaceutical and biotech companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize product candidates without strategic partners or licensees include:
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| ● | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to use our products; |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
We will rely on the availability of specific off-the-shelf components.
We intend to use in part, off-the-shelf components in our product candidates. We may also rely on additional off- the-shelf components for our product candidates or other products. If the supply of any specific off-the-shelf components for our products is terminated due to (i) failure to obtain regulatory approval for the use of such off- the-shelf component, (ii) market demand or (iii) by choice of the manufacturer, then our clinical trials, production, and ultimately, revenue, could be negatively impacted. In addition, our resources would need to be diverted to locate a replacement component, which must then be rigorously tested and qualified. If we fail to have the ability to seamlessly switch from a non-available component to a replacement, then our business, financial condition and results of operations would be materially harmed.
We may have conflicts with our partners that could delay or prevent the development or commercialization of our product candidates.
We may have conflicts with our partners, such as conflicts concerning the interpretation of preclinical or clinical data, the achievement of milestones, the interpretation of contractual obligations, payments for services, development obligations or the ownership of intellectual property developed during our collaboration. If any conflicts arise with any of our partners, such partner may act in a manner that is adverse to our best interests. Any such disagreement could result in one or more of the following, each of which could delay or prevent the development or commercialization of our product candidates, and in turn prevent us from generating revenues: unwillingness on the part of a partner to pay us milestone payments or royalties we believe are due to us under a collaboration; uncertainty regarding ownership of intellectual property rights arising from our collaborative activities, which could prevent us from entering into additional collaborations; unwillingness by the partner to cooperate in the development or manufacture of the product, including providing us with product data or materials; unwillingness on the part of a partner to keep us informed regarding the progress of its development and commercialization activities or to permit public disclosure of the results of those activities; initiating of litigation or alternative dispute resolution options by either party to resolve the dispute; and attempts by either party to terminate the agreement.
35
If we market any of our products in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties and significant civil liability.
The FDA and other government authorities enforce laws and regulations that require that the promotion of pharmaceutical products be consistent with the approved prescribing information. While physicians may prescribe an approved product for a so-called “off-label” use, it is unlawful for a pharmaceutical company to promote its products in a manner that is inconsistent with its approved label and any company which engages in such conduct may be subject to significant liability. Similarly, industry codes in the European Union and other foreign jurisdictions prohibit companies from engaging in off-label promotion and regulatory agencies in various countries enforce violations of the code with civil penalties. While we intend to ensure that our promotional materials are consistent with our label, regulatory agencies may disagree with our assessment and may issue untitled letters, warning letters or may institute other civil or criminal enforcement proceedings. In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include the U.S. Federal Healthcare Program Anti-Kickback Statute, U.S. False Claims Act and similar state laws. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
The U.S. Federal Healthcare Program Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not, in all cases, meet all of the criteria for safe harbor protection from anti-kickback liability. Moreover, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amends the intent requirement of the U.S. Federal Healthcare Program Anti-Kickback Statute and criminal health care fraud statutes; a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the U.S. Federal Healthcare Program Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the U.S. False Claims Act. Federal false claims laws, including the U.S. False Claims Act, impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government.
Over the past few years, pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as: allegedly providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicare or Medicaid for non-covered, off-label uses; using a charity as an illegal conduit to cover the copays of Medicare patients; and submitting inflated best price information to the Medicaid Drug Rebate Program to reduce liability for Medicaid rebates.
Other restrictions under applicable United States federal and state healthcare laws and regulations may include the following:
| ● | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
36
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health, or HITECH Act, and its implementing regulations, also imposes obligations, including mandatory contractual terms, on certain types of people and entities with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | federal transparency laws, including the PSA created under Section 6002 of the Affordable Care Act and its implementing regulations. The PSA requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare and Medicaid Services, or CMS, information related to payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and requires applicable manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by physicians (as defined above) and their immediate family members; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Our products will face significant competition in the markets for such products, and if they are unable to compete successfully, our business will suffer.
Our product candidates face, and will continue to face, intense competition from large pharmaceutical companies, as well as academic and research institutions. We compete in an industry that is characterized by: (i) rapid technological change, (ii) evolving industry standards, (iii) emerging competition and (iv) new product introductions. Our competitors have existing products and technologies that will compete with our products and technologies and may develop and commercialize additional products and technologies that will compete with our products and technologies. Because several competing companies and institutions have greater financial resources than us, they may be able to: (i) provide broader services and product lines, (ii) make greater investments in research and development and (iii) carry on larger research and development initiatives. Our competitors also have greater development capabilities than we do and have substantially greater experience in undertaking preclinical and clinical testing of products, obtaining regulatory approvals, and manufacturing and marketing pharmaceutical products. They also have greater name recognition and better access to customers than us.
Numerous treatments are already established that will compete with our planned commercial therapies, and others are under development. Our competitors may introduce new products that render all or some of our technologies and products obsolete or noncompetitive. In addition, we may not compete successfully against generic competitors. Legislation such as the 21st Century Cures Act, which was enacted in December 2016 and designed to encourage innovation and bring pharmaceutical products to market more quickly, may enable our competitors to bring competing products to market on an expedited basis. In addition, alternative approaches to treating chronic diseases, such as gene therapy, cell therapy or transplantation technologies, may make our products obsolete or noncompetitive.
After approval, if obtained, our products will remain subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional risk and expense, including the FDA withdrawing or modifying approval for our products and product recalls that could harm our reputation, business and financial results.
Drug products remain subject to FDA jurisdiction after they have been approved. Even if we obtain regulatory approval of our product candidates or other products, the FDA may impose significant restrictions on its indicated uses or marketing or the conditions of approval, or impose ongoing requirements for potentially costly and time- consuming post-approval trials, including Phase 4 clinical trials, and post-market surveillance to monitor safety and efficacy. Our product candidates, if approved, will be subject to ongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, sampling, recordkeeping and reporting of adverse events and other post-market information. These requirements include registration with the FDA and continued compliance with cGMPs, and current Good Clinical Practices requirements, for any clinical trials that we conduct post approval.
37
In addition, once a product receives FDA approval, products may be subject to recall for various reasons, including adverse effects, impurities or other product contamination, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.
We may be adversely affected by a failure or compromise from a cyberattack or data breach, which could have an adverse effect on our business.
Companies are subject to a wide variety of cybersecurity attacks on their information technology systems, which we use to maintain proprietary and confidential information. We, our key business partners and third-party suppliers rely on information technology (“IT”) systems to perform business operations, including processing, transmitting and storing electronic information, and interacting with customers, suppliers, healthcare payers, and other third parties. Like other companies in the biopharmaceutical fields, the size and complexity of our IT systems will make them vulnerable to a cyber-attack, malicious intrusion, breakdown, destruction, loss of data privacy, or other significant disruption. Our IT systems will require an ongoing commitment to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information, and changing customer patterns.
Any failure by us to maintain or protect our information technology systems and data integrity related to our products, including from cyber-attacks, intrusions or other breaches, could result in the unauthorized access to patient data and personally identifiable information, theft of intellectual property or other misappropriation of assets, or otherwise compromise the health of patients using our products, our confidential or proprietary information and disrupt our operations.
In the United States, federal and state privacy and security laws require certain of our operations to protect the confidentiality of personal information including patient medical records and other health information. In Europe, the Data Protection Directive will require us to manage individually identifiable information in the European Union and, the new General Data Protection Regulation may impose fines of up to 4% of our global revenue in the event of violations. Internationally, some countries have also passed laws that require individually identifiable data on their citizens to be maintained on local servers and that may restrict transfer or processing of that data. We believe that we will meet the expectations of applicable regulations and that the ongoing costs of compliance with such rules will not be material to our business. However, there is no guarantee that we will be able to comply with these regulations, or otherwise avoid the negative reputational and other affects that might ensue from a significant data breach or failure to comply with applicable data privacy regulations, each of which could have significant adverse effects on our business, financial condition or results of operations.
We may undertake international operations, which will subject us to risks inherent with operations outside of the United States.
We intend to seek to obtain market clearances in foreign markets that we deem to generate significant opportunities. However, even with the cooperation of a commercialization partner, conducting drug development in foreign countries involves inherent risks, including, but not limited to: difficulties in staffing, funding and managing foreign operations; unexpected changes in regulatory requirements; export restrictions; tariffs and other trade barriers; difficulties in protecting, acquiring, enforcing and litigating intellectual property rights; fluctuations in currency exchange rates; and potentially adverse tax consequences.
38
If we were to experience any of the difficulties listed above, or any other difficulties, any international development activities and our overall financial condition may suffer and cause us to reduce or discontinue our international development and registration efforts.
We may not be successful in hiring and retaining key employees.
Our future operations and successes depend in large part upon the continued service of Michael J. Roberts, our President and Chief Executive Officer, and Simon C. Pedder, our Executive Chairman. If these executives terminate their employment with us, such a departure may have a material adverse effect on our business.
Our future success also depends on our ability to identify and recruit prospective executives with proven experience in the biopharmaceutical industry, specifically candidates who have managed and completed FDA-required submissions and clinical trials concerning new products; however, there can be no assurance that such personnel will be available to us or, that once engaged, will be retained by us. Our management team has expertise in many different aspects of drug development and commercialization. However, we will need to hire additional personnel as we further develop the BRiTE platform and product candidates.
Competition for skilled personnel in our market is intense and competition for experienced scientists may limit our ability to hire and retain highly qualified personnel on acceptable terms. Despite our efforts to retain valuable employees, members of our management, scientific and medical teams may terminate their employment with us on short notice. The loss of the services of any of our executive officers or other key employees could potentially harm our business, operating results or financial condition. In particular, we believe that the loss of the services of these or other personnel, would have a material adverse effect on our business. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level, and senior managers as well as junior, mid-level, and senior scientific and medical personnel. Other biopharmaceutical companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer operating history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we have to offer. If we are unable to establish, attract and retain high-quality personnel, the rate and success at which we can develop and commercialize product candidates would be limited and would have a material adverse effect on our business and results of operations.
In addition, the Sarbanes-Oxley Act and related rules implemented by the SEC impose certain requirements on the corporate governance practices of public companies. As a public company, we expect these new rules and regulations to increase our compliance costs in 2025 and beyond and to make certain activities more time consuming and costly. As a public company, we also expect that these rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance in the future and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
Managing our growth as we expand operations may strain our resources.
We intend to grow rapidly in order to support additional, larger, and potentially international, pivotal clinical trials of our drug candidates, which will place a significant strain on our financial, managerial and operational resources. In order to achieve and manage growth effectively, we must continue to improve and expand our operational and financial management capabilities. Moreover, we will need to increase staffing and to train, motivate and manage our employees. All of these activities will increase our expenses and may require us to raise additional capital sooner than expected. Failure to manage growth effectively could harm our business, financial condition or results of operations.
39
Risks Related to Our Intellectual Property
We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our current and future licensors.
Our rights to significant parts of the technology we use in our product candidates are licensed from a third party and are subject to termination if we do not fulfill our contractual obligations to our licensor. Termination of intellectual property rights under our current and future license agreements could adversely impact our ability to produce or protect our products or candidates. Our obligations under our current and future license agreements may include requirements that we make milestone payments to our licensors upon the achievement of clinical development and regulatory approval milestones, royalties as we sell commercial products, and reimbursement of patent filing and maintenance expenses. Should we become bankrupt or otherwise unable to fulfill our contractual obligations, our licensors could terminate our rights to critical technology that we rely upon.
Our ability to protect and enforce any patents we may obtain does not guaranty that we will secure the right to commercialize such patents.
A patent is a limited monopoly right conferred upon an inventor, and his or her successors in title, in return for the making and disclosing of a new and non-obvious invention. This monopoly is of limited duration but, while in force, allows the patent holder to prevent others from making and/or using his or her invention. While a patent gives the holder this right to exclude others, it is not a license to commercialize the invention, where other permissions may be required for permissible commercialization to occur. For example, a drug cannot be marketed without the appropriate authorization from the FDA, regardless of the existence of a patent covering the product. Further, the invention, even if patented itself, cannot be commercialized if it infringes the valid patent rights of another party.
If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish, and our business and competitive position would suffer.
Our success, competitive position and future revenues will depend in part on our ability and the abilities of our licensor to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. We plan to have an active patent protection program that includes filing patent applications on new products or candidates, formulations, delivery systems and methods of making and using products or candidates and prosecuting these patent applications in the United States and abroad. As patents issue, we also file continuation and provisional applications as appropriate. Although we have taken steps to build what we believe to be a strong patent portfolio, we cannot predict:
| ● | the degree and range of protection any patents will afford us against competitors, including whether third parties are able to invalidate or circumvent our licensed patents; |
| ● | if and when patents will issue in the United States or any other country; |
| ● | whether or not others will obtain patents claiming aspects similar to those covered by our licensed patents and patent applications; |
| ● | whether we will need to initiate litigation or administrative proceedings to protect our intellectual property rights, which may be costly whether we win or lose; |
| ● | whether any of the patents we have licensed or may own or license in the future will be challenged by our competitors alleging invalidity or unenforceability and, if opposed or litigated, the outcome of any administrative or court action as to patent validity, enforceability or scope; |
| ● | whether a competitor will develop similar products that are outside the scope of protection afforded by our licensed patents, for example, due to interpretation of claim scope by a court; |
| ● | whether there were activities previously undertaken by our partners that could limit the scope, validity or enforceability of licensed patents and intellectual property; or |
| ● | whether a competitor will assert infringement of its patents or intellectual property, whether or not meritorious, and what the outcome of any related litigation or challenge may be. |
Our success also depends upon the skills, knowledge and experience of our scientific and technical personnel, our consultants and advisors as well as our licensor and contractors. To help protect our proprietary know-how and our inventions for which patents may be unobtainable or difficult to obtain, we intend to rely on trade secret protection and confidentiality agreements. To this end, we require all employees, consultants and directors to enter into agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. These agreements may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information. If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know- how and other proprietary rights would be significantly impaired, and our business and competitive position would suffer.
40
Due to legal and factual uncertainties regarding the scope and protection afforded by patents and other proprietary rights, we may not have meaningful protection from competition.
Our long-term success will substantially depend upon our ability to protect our proprietary technologies from infringement, misappropriation, discovery and duplication and avoid infringing the proprietary rights of others. Our licensed patent rights, like the patent rights of biopharmaceutical companies in general, are highly uncertain and include complex legal and factual issues. These uncertainties also mean that any patents that we may obtain or license in the future could be subject to challenge, and even if not challenged, may not provide us with meaningful protection from competition. Our licensed patents and pending applications may become subject to dispute, and those disputes could be resolved against us.
Any inability to protect intellectual property rights in the United States and foreign countries could limit our ability to manufacture or sell products.
We will rely on patent protection, in some cases trade secrets, unpatented proprietary know-how, and continuing technological innovation to preserve our competitive position. Our patents and licensed patent rights may be challenged, invalidated, infringed or circumvented, and the rights granted in those patents may not provide proprietary protection or competitive advantages to us. We and our licensor may not be able to develop patentable products with acceptable patent protection. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us. If patents containing competitive or conflicting claims are issued to third parties, we may be prevented from commercializing the products covered by such patents, or may be required to obtain or develop alternate technology. In addition, other parties may duplicate, design around or independently develop similar or alternative technologies.
We may not be able to prevent third parties from infringing or using our intellectual property, and the parties from whom we may license intellectual property may not be able to prevent third parties from infringing or using the licensed intellectual property. We plan to control and limit access to, and the distribution of, our product documentation and other proprietary information. Despite efforts to protect this proprietary information, unauthorized parties may obtain and use information that we may regard as proprietary. Other parties may independently develop similar know-how or may even obtain access to these technologies.
The laws of some foreign countries do not protect proprietary information to the same extent as the laws of the United States, and many companies have encountered significant problems and costs in protecting their proprietary information in these foreign countries.
Neither the U.S. Patent and Trademark Office nor the courts have established a consistent policy regarding the breadth of claims allowed in pharmaceutical and biotechnology patents. The allowance of broader claims may increase the incidence and cost of patent administrative proceedings and the risk of infringement litigation. On the other hand, the allowance of narrower claims may limit the value of our proprietary rights.
If some or all of our licensed patents expire or are invalidated or are found to be unenforceable, or if some or all of our licensed patent applications do not result in issued patents or result in patents with narrow, overbroad, or unenforceable claims, or claims that are invalidated for, for example not being supported in regard to written description or enablement by the specification, or if we are prevented from asserting that the claims of an issued patent cover a product of a third party or its use, we may be subject to competition from third parties with products in the same class of products as our product candidates or products with the same product platform as our product candidates, including in those jurisdictions in which we have no patent protection.
Our commercial success will depend in part on obtaining and maintaining patent and trade secret protection for our product candidates, as well as the methods for using these product candidates, and the methods for producing our product candidates. We will be able to protect our product candidates and the methods for using these product candidates from unauthorized use by third parties only to the extent that we or our exclusive licensor owns or controls such valid and enforceable patents or trade secrets.
41
Even if our product candidates and the methods for using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Our and any licensor’s abilities to obtain patents can be highly uncertain and involve complex and in some cases unsettled legal issues and factual questions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries provide different degrees of protection against the use of a patented invention by others. Therefore, if a patent covers an invention issues in a given country and no patents covering the same invention issued in other countries, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the utility, written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection.
We may be subject to competition from third parties with products in the same class of products as our product candidates, or products with the same product platform as our product candidates in those jurisdictions in which we have no patent protection. Even if patents are issued to us or any licensor regarding our product candidates or methods of using and making them, those patents can be challenged by our competitors as invalid or unenforceable on a variety of grounds, including lack of sufficient written description or enablement, lack of utility, lack of novelty and inventiveness, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect our product candidates if competitors devise ways of making or using these products without legally infringing our patents. The current United States regulatory environment may have the effect of encouraging companies to challenge branded drug patents or to create non-infringing versions of a patented product in order to facilitate the approval of abbreviated new drug applications for generic substitutes. These same types of incentives encourage competitors to submit new drug applications that rely on literature and clinical data not prepared for or by the drug sponsor, providing another less burdensome pathway to approval.
We rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties, our trade secrets may become known to our competitors.
We rely on trade secrets that we seek to protect through confidentiality agreements with our employees and other parties. If these agreements are breached, our competitors may obtain and use our trade secrets to gain a competitive advantage over us. We may not have any remedies against our competitors and any remedies that may be available to us may not be adequate to protect our business or compensate us for the damaging disclosure. In addition, we may have to expend significant resources to protect our interests from possible infringement by others.
If we or our third-party suppliers are found to be infringing on patents or trade secrets owned by others, we may be forced to cease or alter our product development efforts, obtain a license to continue the development or sale of our products, and/or pay damages.
As the pharmaceutical industry expands and more patents are issued, the risk increases that our or our third-party suppliers’ processes and potential products may give rise to claims that they infringe the patents, trademarks, copyrights, trade secrets, or other intellectual property rights of others. Although we have reviewed certain third- party patents and patent filings that we believe may be relevant to our therapeutic candidates or products, we have not conducted any freedom-to-operate search or analysis for our therapeutic candidates or products, and we may not be aware of patents or pending or future patent applications that, if issued, would block us from commercializing our therapeutic candidates or products. Thus, we cannot guarantee that our therapeutic candidates or products, or our commercialization thereof, do not and will not infringe any third party’s intellectual property. We may, from time to time, be notified of claims that we or our third party suppliers are infringing upon patents, trademarks, copyrights, trade secrets or other intellectual property rights owned by third parties, including potential competitors, and we cannot provide assurances that other companies will not, in the future, pursue such infringement claims against us or any third-party proprietary technologies we have licensed. If we or our third party suppliers were found to infringe upon a patent or other intellectual property right, or if we failed to obtain or renew a license under a patent or other intellectual property right from a third party, or if a third party that we were licensing technologies from was found to infringe upon a patent or other intellectual property rights of another third party, we may be required to pay damages. These other persons could also bring legal actions against us, or our third-party suppliers, claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the affected product or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to conduct clinical tests, manufacture or market the affected product or use the affected process. Required licenses may not be available on acceptable terms, if at all, and the results of litigation are uncertain. If we become involved in litigation or other proceedings, it could consume a substantial portion of our financial resources and the efforts of our personnel. If our suppliers become involved in litigation or other proceedings, it could prevent us from continuing to develop or commercialize our products. Additionally, rights granted under licensing agreements may not provide a competitive advantage to us. Efforts to enforce patent rights can involve substantial expense and may not be successful. Furthermore, others may independently develop similar, superior, or parallel technologies to any technology developed or licensed by us, or our technology may prove to infringe on patents or rights owned by others. Thus the patents licensed to us, or in the future licensed or held by us, may not afford us any meaningful competitive advantage. Also, our confidentiality agreements may not provide meaningful protection of our proprietary information. Our inability to maintain our proprietary rights could have a material adverse effect on our business, financial condition, and results of operations.
42
Other parties may claim that we infringe their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling products.
Our success will depend in part on our ability to operate without infringing the patents and proprietary rights of third parties. The development, manufacture, use and sale of new products have been subject to substantial patent rights litigation in the pharmaceutical and biotech industry. These lawsuits generally relate to the validity and infringement of patents or proprietary rights of third parties. Infringement litigation is prevalent with respect to generic versions of products for which the patent covering the brand name product is expiring, particularly since many companies that market generic products focus their development efforts on products with expiring patents. Pharmaceutical companies, biotechnology companies, universities, research institutions or other third parties may have filed patent applications or may have been granted patents that cover aspects of our product candidates or other technologies.
Future or existing patents issued to third parties may contain patent claims that cover our products or candidates. We expect to be subject to infringement claims from time to time in the ordinary course of business, and third parties could assert infringement claims against us in the future with respect to our current product candidates or with respect to product candidates that we may develop or license. Litigation or administrative proceedings could force us to:
| ● | stop or delay selling, manufacturing or using products that incorporate, or are made using the challenged intellectual property; |
| ● | pay damages; or |
| ● | enter into licensing or royalty agreements that may not be available on acceptable terms, if at all. |
Any litigation or administrative proceedings, regardless of their outcome, would likely delay the regulatory approval process, be costly and require significant time and attention of our key management and technical personnel.
We may be subject to claims that our consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, we engage the services of consultants to assist us in the development of our product candidates. Many of these consultants were previously employed at, or may have previously or may be currently providing consulting services to, other biotechnology or pharmaceutical companies including our competitors or potential competitors. We may become subject to claims that the Company or a consultant inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management team.
43
Risks Related to Ownership of our Common Stock
If we are unable to register in a timely manner the shares of Common Stock issued to stockholders in the Merger or the Offering, then we may be subject to certain liquidated damages.
We have agreed to prepare and file with the SEC a registration statement registering the resale of shares sold in the Offering. If (a) we are late in filing the resale registration statement, (b) the resale registration statement is not declared effective within 120 days after the final closing of the Offering (subject to certain exceptions), (c) the resale registration statement ceases for any reason to remain effective or the holders of shares are otherwise not permitted to utilize the prospectus therein to resell their shares for a period of more than five consecutive trading days (subject to permitted blackout periods (as defined in the Registration Rights Agreement) and certain other exceptions), or (d) following the listing or inclusion for quotation of the Common Stock on OTC Markets, Nasdaq, NYSE or NYSE American, the shares are not listed or included for quotation on such a market, or trading of the Common Stock is suspended or halted on the principal market for the Common Stock, for more than three consecutive trading days (subject to certain exceptions), then in any such case monetary penalties payable by the Company to the affected holders of the shares will commence to accrue at a rate equal to 12% per annum of the value of the Registrable Shares, as set forth in the Registration Rights Agreement. If we are required to pay the holders of the Registrable Shares a significant cash penalty our financial condition may be harmed.
There is currently no market for our Common Stock and there can be no assurance that any market will ever develop.
Our Common Stock is not listed on a national securities exchange or any other exchange, or quoted on an over-the- counter market. Therefore, there is no trading market, active or otherwise, for our Common Stock and our Common Stock may never be included for trading on any stock exchange, automated quotation system or any over-the-counter market. Accordingly, our Common Stock is highly illiquid and our investors will likely experience difficulty in selling their shares at times and prices that they may desire.
Our Common Stock may not be eligible for listing or quotation on any securities exchange or over-the-counter trading system.
We do not currently meet the initial quantitative listing standards of any national securities exchange or over-the- counter trading system. We cannot assure you that we will be able to meet the initial listing standards of any national securities exchange or over-the-counter trading system, or, if we do meet such initial listing standards, that we will be able to maintain any such listing. Further, will be required to meet certain requirements, including prescribed periods of time trading over-the-counter and minimum filings of periodic reports with the SEC, before we are eligible to apply for listing on a national securities exchanges. We intend to contact an authorized market maker for an over-the-counter quotation system for sponsorship of our Common Stock, but we cannot guarantee that such sponsorship will be approved and our Common Stock listed and quoted for sale. Even if our Common Stock is quoted for sale on an over-the-counter quotation system, buyers may be insufficient in numbers to allow for a robust market and it may prove impossible to sell your shares. In addition, an investor may find it difficult to obtain accurate quotations as to the market value of our Common Stock. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our Common Stock, which may further affect its liquidity. This would also make it more difficult for us to raise additional capital.
44
The market price and trading volume of our Common Stock may be volatile and could decline significantly.
The quotation systems, including the OTC Markets QB tier and QX tier, or stock exchanges, including Nasdaq, on which our Common Stock may be quoted or on which our Common Stock may be listed in the future have from time to time experienced significant price and volume fluctuations. Even if an active, liquid and orderly trading market develops and is sustained for our Common Stock following the Merger, the market price of our Common Stock may be volatile and could decline significantly. In addition, the trading volume in our Common Stock may fluctuate and cause significant price variations to occur. If the market price of our Common Stock declines significantly, our stockholders may be unable to resell their shares at or above the market price of our Common Stock as of the date they purchased them. We cannot assure you that the market price of our Common Stock will not fluctuate widely or decline significantly in the future in response to a number of factors, including, among others, the following:
| ● | the realization of any of the risk factors presented in this Report; |
| ● | future issuances, sales, resales or repurchases or anticipated issuances, sales, resales or repurchases, of our Common Stock; |
| ● | actual or anticipated differences in our estimates, or in the estimates of analysts, for our revenues, results of operations, level of indebtedness, liquidity or financial condition; |
| ● | additions and departures of key personnel; |
| ● | failure to comply with the requirements of the OTCQB market, or following our potential up listing on Nasdaq; |
| ● | failure to comply with the Sarbanes-Oxley Act or other laws or regulations; |
| ● | publication of research reports about us, or our industry; |
| ● | the performance and market valuations of other similar companies; |
| ● | broad disruptions in the financial markets, including sudden disruptions in the credit markets; |
| ● | speculation in the press or investment community; |
| ● | actual, potential or perceived control, accounting or reporting problems; and |
| ● | changes in accounting principles, policies and guidelines. |
In the past, securities class-action litigation has often been instituted against companies following periods of volatility in the market price of their shares. This type of litigation could result in substantial costs and divert our management’s attention and resources, which could have a material adverse effect on us.
The designation of our Common Stock as “penny stock” would limit the liquidity of our Common Stock.
Our Common Stock may be deemed a “penny stock” (as that term is defined under Rule 3a51-1 of the Exchange Act) in any market that may develop in the future. Generally, a “penny stock” is a common stock that is not listed on a securities exchange and trades for less than $5.00 a share. Prices often are not available to buyers and sellers and the market may be very limited. Penny stock in start-up companies is among the riskiest equity investments. Broker-dealers who sell penny stock must provide purchasers with a standardized risk-disclosure document prepared by the SEC. The document provides information about penny stock and the nature and level of risks involved in investing in the penny stock market. A broker must also provide purchasers with bid and offer quotations and information regarding broker and salesperson compensation and make a written determination that the penny stock is a suitable investment for the purchaser and obtain the purchaser’s written agreement to the purchase. Many brokers choose not to participate in penny stock transactions. If our Common Stock is deemed “penny stock”, because of penny stock rules, there may be less trading activity in any market that develops for our Common Stock in the future and stockholders are likely to have difficulty selling their shares.
FINRA sales practice requirements may limit a stockholder’s ability to buy and sell our Common Stock.
The Financial Industry Regulatory Authority, or FINRA, has adopted rules requiring that, in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative or low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA has indicated its belief that there is a high probability that speculative or low-priced securities will not be suitable for at least some customers. If these FINRA requirements are applicable to us or our securities, they may make it more difficult for broker-dealers to recommend that at least some of their customers buy our Common Stock, which may limit the ability of our stockholders to buy and sell our Common Stock and could have an adverse effect on the market for and price of our Common Stock.
45
Because we became a reporting company under the Exchange Act by means other than a traditional underwritten initial public offering, we may not be able to attract the attention of research analysts at major brokerage firms.
Because we will become a reporting company by conducting an underwritten initial public offering of our Common Stock, and because we will not be listed on a national securities exchange, security analysts of brokerage firms may not provide coverage of our Company. In addition, investment banks may be less likely to agree to underwrite secondary offerings on our behalf than they might if we became a public reporting company by means of an underwritten initial public offering, because they may be less familiar with our Company as a result of more limited coverage by analysts and the media, and because we became public at an early stage in our development. The failure to receive research coverage or support in the market for our shares will have an adverse effect on our ability to develop a liquid market for our Common Stock.
Because the Merger was a reverse merger, the Registration Statement we file with respect to the shares of Common Stock received by investors in the Merger might be subject to heightened scrutiny by the SEC, and we may not be able to attract the attention of major brokerage firms.
Additional risks may exist as a result of our becoming a public reporting company through a “reverse merger.” Certain SEC rules are more restrictive when applied to reverse merger companies, such as the ability of stockholders to resell their shares of Common Stock pursuant to Rule 144, and the SEC may subject the Registration Statement we file with respect to the shares of Common Stock received by investors in the Merger and the Offering to heightened scrutiny. In addition, securities analysts of major brokerage firms may not provide coverage of our capital stock or business. Because we became a public reporting operating company through a reverse merger, there is no incentive to brokerage firms to recommend the purchase of our Common Stock. We cannot assure you that brokerage firms will want to provide analyst coverage of our capital stock or business in the future.
We are obligated to develop and maintain proper and effective internal control over financial reporting. If we fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable laws and regulations could be impaired. In addition, the presence of material weaknesses increases the risk of material misstatement of the consolidated financial statements.
Pursuant to Section 404(a) of the Sarbanes-Oxley Act, we are required to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting in our Annual Report on Form 10-K. Effective internal control over financial reporting is necessary for reliable financial reports and, together with adequate disclosure controls and procedures, such internal controls are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. Ineffective internal controls could also cause investors to lose confidence in reported financial information, which could have a negative effect on the trading price of our Common Stock.
The report by management will need to include disclosure of any material weaknesses identified in internal control over financial reporting. However, for as long as we are an “emerging growth company” under the JOBS Act following the consummation of the Merger, our independent registered public accounting firm will not be required to attest to the effectiveness of internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act.
Management’s assessment of internal controls, when implemented, could detect problems with internal controls, and an independent assessment of the effectiveness of internal controls by our auditors could detect further problems that management’s assessment might not, and could result in the identification of material weaknesses that were not otherwise identified. For example, in connection with its audit of Private Adaptin’s financial statements as of and for the years ended December 31, 2023 and 2022, Private Adaptin’s external auditor identified certain material weaknesses in its internal control over financial reporting with respect to the following items:
| ● | insufficient evidence of board preapproval before entering into debt, equity, and licensing agreements; |
| ● | errors in accounting for non-routine transactions; and |
| ● | segregation of duties. |
46
Private Adaptin’s management intends to take measures to remediate the aforementioned material weaknesses, including, but not limited to the engagement of a professional services firm to provide back-office accounting support, including the services of a corporate controller; and consulting support with respect to the application of Generally Accepted Accounting Principles and SEC financial reporting regulations. The material weaknesses will not be considered remediated until management designs and implements effective controls that operate for a sufficient period of time and management has concluded, through testing, that these controls are effective. Management will monitor the effectiveness of these remediation plans and will make changes management determines to be appropriate. There can be no assurance that any remediation plans will be successful.
Undetected material weaknesses in internal controls, or those that are detected and not timely resolved, could lead to financial statement restatements and require us to incur the expense of remediation. We are required to disclose changes made in internal control and procedures on a quarterly basis. To comply with the public company requirements, we may need to undertake various actions, such as implementing new internal controls and procedures and hiring accounting or internal audit staff.
We are in the early stages of developing the system and processing documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete its evaluation, testing, and any required remediation in a timely fashion. During the evaluation and testing process, if we cannot remedy any identified material weaknesses or if we identify additional material weaknesses in internal control over financial reporting, we will be unable to assert that internal control over financial reporting is effective.
If we are unable to assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of its internal control, including as a result of any new or existing material weaknesses described above, we could lose investor confidence in the accuracy and completeness of financial reports, which would cause the price of our Common Stock to decline, and we may be subject to investigation or sanctions by the SEC. In addition, if we are unable to continue to meet these requirements, we may not be able to remain quoted on any over-the-counter trading system, or following any potential listing, listed on any securities exchange.
We are an emerging growth company and a smaller reporting company, and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to emerging growth companies and smaller reporting companies could make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and, for as long as we continue to be an emerging growth company, we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to emerging growth companies, including:
| ● | not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act; |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports and annual report on Form 10-K; and |
| ● | exemptions from the requirements of holding non-binding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
Our status as an emerging growth company will end upon the earlier of: (a) the fifth anniversary of the first sale of our common equity securities pursuant to an effective registration statement; (b) the last day of the fiscal year in which we have more than $1.235 billion in annual gross revenues; (c) the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; and (d) the date on which we have issued, in any three-year period, more than $1 billion in non-convertible debt securities.
We cannot predict if investors will find our Common Stock less attractive if we choose to rely on any of the exemptions afforded emerging growth companies. If some investors find our Common Stock less attractive because we rely on any of these exemptions, there may be a less active trading market for our Common Stock and the market price of our Common Stock may be more volatile.
47
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to avail ourselves of this provision of the JOBS Act. As a result, we will not be subject to new or revised accounting standards at the same time as other public companies that are not emerging growth companies. Therefore, our consolidated financial statements may not be comparable to those of companies that comply with new or revised accounting pronouncements as of public company effective dates.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a “smaller reporting company” even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as our voting and non-voting Common Stock held by non-affiliates is less than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues is less than $100 million during the most recently completed fiscal year and our voting and non-voting Common Stock held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter.
We may face risks related to securities litigation that could result in significant legal expenses and settlement or damage awards.
We may in the future become subject to claims and litigation alleging violations of the securities laws or other related claims, which could harm our business and require us to incur significant costs. Significant litigation costs could impact our ability to comply with certain financial covenants under our credit agreement. We are generally obliged, to the extent permitted by law, to indemnify our current and former directors and officers who are named as defendants in these types of lawsuits. Regardless of the outcome, litigation may require significant attention from management and could result in significant legal expenses, settlement costs or damage awards that could have a material impact on our financial position, results of operations and cash flows.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Our restated certificate of incorporation and our restated bylaws contain provisions that could delay or prevent a change in control of our company. These provisions could also make it difficult for stockholders to elect directors who are not nominated by current members of our board of directors or take other corporate actions, including effecting changes in our management. These provisions:
| ● | permit only the board of directors to establish the number of directors and fill vacancies on the board; | |
| ● | require super-majority voting to amend some provisions in our restated certificate of incorporation and restated bylaws; | |
| ● | authorize the issuance of “blank check” preferred stock that our board of directors could use to implement a stockholder rights plan; | |
| ● | eliminate the ability of our stockholders to call special meetings of stockholders; | |
| ● | prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders; | |
| ● | prohibit cumulative voting; and | |
| ● | establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon by stockholders at annual stockholder meetings. |
In addition, our restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for: any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law (the “DGCL”), our restated certificate of incorporation, or our restated bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine.
48
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all claims brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Our restated certificate of incorporation provides that the federal district courts of the United States is, unless we consent in writing to an alternative forum, the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act (“Federal Forum Provision”). Our decision to adopt a Federal Forum Provision followed a decision by the Supreme Court of the State of Delaware holding that such provisions are facially valid under Delaware law. While there can be no assurance that federal courts or state courts will follow the holding of the Delaware Supreme Court or determine that the Federal Forum Provision should be enforced in a particular case, application of the Federal Forum Provision means that suits brought by our stockholders to enforce any duty or liability created by the Securities Act must be brought in federal court and cannot be brought in state court. While neither the exclusive forum provision nor the Federal Forum Provision applies to suits brought to enforce any duty or liability created by the Exchange Act creates exclusive federal jurisdiction over all claims brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Accordingly, actions by our stockholders to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder also must be brought in federal court. Our stockholders are not deemed to have waived our compliance with the federal securities laws and the regulations promulgated thereunder.
Any person or entity purchasing or otherwise acquiring or holding any interest in any of our securities is deemed to have notice of and consented to our exclusive forum provisions, including the Federal Forum Provision. These provisions may limit a stockholder’s ability to bring a claim in a judicial forum of their choosing for disputes with us or our directors, officers or other employees, which may discourage lawsuits against us and our directors, officers, and other employees.
In addition, Section 203 of the DGCL may discourage, delay or prevent a change in control of our company. Section 203 imposes certain restrictions on mergers, business combinations and other transactions between us and holders of 15% or more of our Common Stock.
The issuance of our preferred stock could adversely affect the holders of our Common Stock in some circumstances.
The issuance of some or all of our authorized preferred stock could adversely affect the holders of our Common Stock in some circumstances. Our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights, which could adversely affect the voting power, or other rights of the holders of the Common Stock. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of the Company. Although we have no present intention to issue any shares of our authorized preferred stock, there can be no assurance that the Company will not do so in the future.
Because we do not intend to pay dividends, stockholders will benefit from an investment in our Common Stock only if it appreciates in value.
We have never declared or paid any cash dividends on our preferred stock or Common Stock nor do we intend to do so. For the foreseeable future, it is expected that earnings, if any, generated from our operations will be used to finance the growth of our business, and that, other than with respect to dividends we may be obligated to pay on our preferred stock, if any, no dividends will be paid to holders of our Common Stock. As a result, the success of an investment in our Common Stock will depend upon any future appreciation in its value. There is no guarantee that our Common Stock will appreciate in value.
If securities or industry analysts do not publish research or publish unfavorable or inaccurate research about our business, our stock price and trading volume could decline.
Our stock price and trading volume following our quotation on the OTC Markets QB tier, if any, or following our potential listing on a securities exchange, if any, will be heavily influenced by the way analysts and investors interpret our financial information and other disclosures. Securities and industry analysts do not currently, and may never, publish research on our business. If few securities or industry analysts commence coverage of us, our stock price could be negatively affected. If securities or industry analysts downgrade our Common Stock, or publish negative reports about our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our Common Stock could decrease, which might cause our stock price to decline and could decrease the trading volume of our Common Stock.
49
The future issuance of equity or of debt securities that are convertible into equity may dilute our stockholders’ investment and reduce their equity interest.
We may choose to raise additional capital in the future, depending on market conditions, strategic considerations and operational requirements. To the extent that additional capital is raised through the issuance of shares or other securities convertible into shares, our stockholders will be diluted, including to the extent there are additional closings of the Offering. Future issuances of our Common Stock or other equity securities, or the perception that such sales may occur, could adversely affect the prevailing market price of our Common Stock and impair our ability to raise capital through future offerings of equity or equity-linked securities. For example, we have agreed, at our expense, to prepare and file a registration statement with the SEC registering the resale of certain shares of our Common Stock pursuant to the Registration Rights Agreement. See “Completion of Acquisition or Disposition of Assets-The Merger and Related Transactions-Registration Rights” for more information. The resale of a substantial number of shares of our Common Stock in the public market could adversely affect the market price for our Common Stock and make it more difficult for you to sell shares of our Common Stock at times and prices that our stockholders feel are appropriate. Furthermore, we expect that, because there will be a large number of shares registered pursuant to a registration statement, selling stockholders will continue to offer shares covered by such registration statement for a significant period of time, the precise duration of which cannot be predicted. Accordingly, the adverse market and price pressures resulting from an offering pursuant to a registration statement may continue for an extended period of time and continued negative pressure on the market price of our Common Stock could have a material adverse effect on our ability to raise additional equity capital.
We are subject to the reporting requirements of federal securities laws, which can be expensive and may divert resources from other projects, thus impairing our ability to grow.
We are a public reporting company and, accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, including compliance with the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports, proxy statements, registration statements and other information with the SEC and furnishing audited reports to stockholders would cause our expenses to be higher than they would be if Private Adaptin remained privately held and did not consummate the Merger. In addition, we will incur substantial expenses in connection with the preparation of the Registration Statement and related documents required under the terms of the Offering.
It may be time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures. If we are unable to comply with the internal controls requirements of the Sarbanes-Oxley Act, then we may not be able to obtain the independent accountant certifications required by such act, which may preclude us from keeping our filings with the SEC current.
Our principal stockholders will have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company.
The Company’s founders, executive officers, directors, and other principal stockholders, in the aggregate, beneficially own a majority of our outstanding stock. These stockholders will have significant influence with respect to the election of our board of directors and approval or disapproval of all significant corporate actions. The concentrated voting power of these stockholders could have the effect of delaying or preventing an acquisition of the company or another significant corporate transaction.
For example, the sole holder of Unite Acquisition prior to the Merger, Lucius Partners, holds 3,250,000 shares of Common Stock after the Merger, which represents 40.2% of the outstanding shares of the combined Company as of the date of this Report. Lucius Partners purchased its shares upon formation of the Company for a nominal price. Matthew Eitner, the Chief Executive Officer of the Placement Agent, James Ahern, the Managing Partner of the Placement Agent, and Patrick Gallagher, a member of our board of directors, a Managing Director of the Placement Agent, are managing members, members, and/or officers of Lucius Partners, and therefore are indirectly material stakeholders of the Company. Anthony Zook is also a member of the board of directors of the Company and is a member of Lucius Partners. The Placement Agent and/or its designees hold warrants to purchase an aggregate of 270,204 shares of our Common Stock. Therefore, following the Merger, such persons can exert substantial control over matters requiring the approval of our stockholders. Additionally, Lucius Partners has received, and will continue to receive after the Merger, fees from us for advisory and certain other services to the Company.
50
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included in this Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties as described under the heading “Forward-Looking Statements” elsewhere in this Report. You should review the disclosure under the heading “Risk Factors” in this Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
Concurrent with the closing of the Offering, Unite Acquisition, Merger Sub, and Private Adaptin entered into Merger Agreement and effected the Merger. Pursuant to the terms of the Merger Agreement, on the Closing Date, Merger Sub merged with and into Private Adaptin, with Private Adaptin continuing as the surviving corporation and the wholly- owned subsidiary of Unite Acquisition. As a result of the Merger, Unite Acquisition acquired the business of Private Adaptin and will continue the existing business operations of Private Adaptin as a public reporting company under the name “Adaptin Bio, Inc.” On the Closing Date, Private Adaptin was renamed “Adaptin Bio Operating Corporation.”
The Merger is treated as a recapitalization and reverse acquisition for Unite Acquisition for financial reporting purposes, and Private Adaptin is considered the acquirer for accounting purposes. As a result of the Merger and the change in Unite Acquisition’s business and operations, a discussion of the past financial results of Unite Acquisition is not pertinent, and under applicable accounting principles, the historical financial results of Private Adaptin, the accounting acquirer, prior to the Merger will be considered the combined Company’s historical financial results.
Company Overview
Adaptin is a biopharmaceutical company pioneering a transformational approach to enhancing the transfer of therapeutics into the brain, facilitating the treatment of brain cancers and other unmet medical conditions. The Company’s precision medicine technology, originally developed by researchers in the Department of Neurosurgery at Duke University, harnesses the human immune system’s ability to target, recognize, destroy or deliver therapeutics to specific cells, including cancer cells. Our mission is to be the global leader and pioneer of this new treatment paradigm, integrating recombinant technology, gene therapy and cell therapy to address the challenges of targeting and delivering effective therapies, including to the brain for cancer and other CNS, indications.
We are closely working with the researchers at Duke University to translate preclinical proof of concept data of our proprietary platform technology, the BRiTE Platform, into human clinical trials. BRiTE is a translatable method to specifically target malignant glioma using a tumor-specific, fully human bispecific antibody that redirects patients’ own T cells to recognize and destroy tumor cells. Our first application of BRiTE is APTN-101, a proprietary EGFRvIII x CD3 bispecific T cell engager, that is able to eliminate malignant glioma tumors in a variety of aggressive preclinical orthotopic tumor models. We designed APTN-101 to specifically redirect T cells against tumors expressing a well-characterized, mutated form of EGFR on a number of tumor types, including glioblastoma, breast and lung cancer. APTN-101 has been recently accepted under an investigator-led IND to begin first-in-human studies in brain cancer. Our goal is to complete preclinical studies on additional product candidates and file multiple INDs.
Duke University Exclusive Licensing Agreement
As described elsewhere in this Report, effective January 11, 2023, Private Adaptin entered into the Duke License, which was assumed by the Company in the Merger, whereby Duke University granted Private Adaptin an exclusive license to the patents and patent application relating to the precision medicine technology, which we intend to develop using our BRiTE Platform. As part of the consideration for the license, Private Adaptin issued Duke University 75 shares (that were valued at $175.86 per share, or $13,189), representing 5% of its then-issued and outstanding common stock on a fully diluted basis, which converted into 161,960 shares of Common Stock, representing 2.0% of the currently outstanding Common Stock. We are obligated to make milestone payments and pay royalties to Duke University, as well as to reimburse Duke University for prior patent expenses, as set forth elsewhere in this Report.
51
The Company has recorded the issuance of common stock and has accrued for the reimbursement of prior patent expenses, but has not reached any of the clinical or regulatory milestones or transacted its first commercial sale as of September 30, 2024. The Company will record corresponding liabilities as such events occur.
Recent Events
Bridge Financings
Private Adaptin raised bridge financing through the offer and sale (a) in 2023 of $500,000 principal amount of its 2023 Bridge Notes and (b) in 2024 of $1,000,000 principal amount of its 2024 Bridge Notes, which in each case were sold to a limited number of accredited investors pursuant to Regulation D under the Securities Act.
At the closing of the Offering, the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, converted automatically into shares of Common Stock at a conversion price of $3.30 per share, or 501,140 shares of our Common Stock, and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, Pre-Merger Warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of either $3.30 or $4.40 per share and with a term of five years.
Operations Overview
Since the commencement of Private Adaptin’s operations in 2021, we have devoted substantially all of our resources to supporting our product development efforts, raising capital to support and expand such activities, and providing general and administrative support for these operations. We operate our business using a significant outsourcing model. As such, our team is composed of a small group of employees who direct a significantly large number of team members, including vendors and consultants, to enable execution of our operational plans. We do not currently have any products approved for sale, and we will continue to incur significant research and development and general administrative expenses related to our operations.
Since inception, we have incurred significant operating losses. For the nine months ended September 30, 2024 and 2023, we recorded net losses of approximately $2.3 million and $0.7 million, respectively. As of September 30, 2024, we had an accumulated deficit of approximately $3.2 million. We expect to continue to incur significant losses for the foreseeable future. We anticipate that a substantial portion of our capital resources and efforts in the foreseeable future will be focused on completing the necessary development activities required for applying for and obtaining regulatory approval for our product candidates and, subsequently, preparing for potential commercialization of our product candidates. We had approximately $115,000 of cash at September 30, 2024.
We expect to continue to incur significant expenses and operating losses for at least the next several years. Our net losses may fluctuate significantly from period to period, depending on the timing of our planned clinical trials and expenditures on other research and development activities. We expect our expenses will increase substantially over time as we:
| ● | continue our ongoing and planned development of APTN-101, including pre-clinical activity and our Phase 1 investigator-led trial for the treatment of GBM; | |
| ● | build a portfolio of product candidates through development, or the acquisition or in-license of drugs, product candidates or technologies; | |
| ● | initiate preclinical studies and clinical trials for APTN-101 for any additional indications we may pursue and for any additional product candidates that we may pursue in the future; | |
| ● | maintain, protect and expand our intellectual property portfolio; | |
| ● | hire clinical, regulatory and scientific personnel; | |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development efforts; and | |
| ● | incur additional legal, accounting, insurance and other expenses associated with operating as a public company. |
52
The Macroeconomic Climate
The recent economic trends and political changes, including the proposed implementation of a tariff structure, may materially adversely affect our business and corresponding financial position and cash flows. While inflationary factors have trended down and interest rates are beginning to trend down, they still may impact our overhead costs and may adversely affect our operating results. While interest rates have recently been trending down, they remain high and present a challenge impacting the United States and global economies. Such factors could make it more difficult for us to obtain traditional financing on acceptable terms, if at all, in the future. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, we may experience increases in the near future on our operating costs, including our labor, due to supply chain constraints, consequences associated with pandemics or public health situations, the Russia-Ukraine war, and other United States geopolitical issues, such as the proposed tariff structures, affecting other territories and employee availability and wage increases, all of which may result in additional stress on our working capital resources.
Components of Results of Operations
Research and Development Expenses
Research and development expenses consist primarily of fees paid to third-party service providers and, in future periods, we expect will include additional personnel costs and other personnel-related compensation expenses. Research and development expenses also include the cost of in-process research and development (“IPR&D”) assets purchased in an asset acquisition transaction. We have concluded that the costs associated with licensing our technology are IPR&D and we expensed those costs upon execution of the Duke License. Research and development costs after the acquisition are expensed as incurred. We expense research and development costs in the periods in which they are incurred. Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors, collaborators and third-party service providers.
To date, substantially all our research and development expenses have been related to the licensing and preclinical development of APTN-101. As we progress, we expect our research and development costs to increase for additional preclinical and clinical development of APTN-101 in GBM.
The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming and is subject to uncertainties and delays. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenue from the commercialization and sale of our product candidates, if at all.
General and Administrative Expenses
General and administrative expenses include expenses for outside professional services and other general administrative expenses. Outside professional services consist of patent maintenance expenses, legal, accounting and audit services and other consulting fees.
We also expect to incur expenses as a public company, including expenses related to compliance with SEC rules and regulations and those of any national securities exchange on which our securities are traded, additional insurance expenses, investor relations activities, and other administrative and professional services.
Interest Expense
Interest expense primarily consists of contractual debt interest expense, the amortization of debt issuance costs and the amortization of discounts arising from derivative liabilities.
53
Results of Operations
Comparison of the Nine Months Ended September 30, 2024 and 2023
The following table summarizes our results of operations and changes for the periods indicated:
| Nine months ended September 30, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research and development | $ | 1,566,533 | $ | 440,299 | $ | 1,126,234 | 256 | % | ||||||||
| General and administrative | 495,272 | 212,623 | 282,649 | 133 | % | |||||||||||
| Total Operating Expenses | 2,061,805 | 652,922 | 1,408,883 | 216 | % | |||||||||||
| LOSS FROM OPERATIONS | (2,061,805 | ) | (652,922 | ) | (1,408,883 | ) | 216 | % | ||||||||
| Interest Expense | 195,161 | 66,711 | 128,450 | 193 | % | |||||||||||
| Loss on fair value of derivative liability | 8,755 | - | 8,755 | 100 | % | |||||||||||
| NET LOSS | $ | (2,265,721 | ) | $ | (719,633 | ) | $ | (1,546,088 | ) | 215 | % | |||||
Research and Development Expenses
Research and development expenses increased by $1,126,234, or 256%, for the nine months ended September 30, 2024 when compared to research and development expenses for the nine months ended September 30, 2023. During 2024, the increase in research and development costs are primarily associated with a repeat-dose toxicology study of APTN-101 that began in late 2023. Costs incurred during the nine months ended September 30, 2023 consist primarily of costs related to the execution of the Duke License that was determined to be IPR&D acquired in an asset transaction. These costs were expensed as the technology has not received regulatory approval for marketing and, absent obtaining such approval, has no established alternative future use.
General and Administrative Expenses
General and administrative expenses increased by $282,649, or 133%, for the nine months ended September 30, 2024 when compared to the nine months ended September 30, 2023. The increase was primarily related to increases in consulting costs related to management of the Company as business activities increased.
Interest Expense
Interest expense increased $128,450 for the nine months ended September 30, 2024 when compared to the nine months ended September 30, 2023. The increase in interest expense was related to issuance of $1.0 million in 2024 Notes and the interest accrued, debt issuance costs amortization, discounts related to derivative liability amortization and related costs. In addition, we recorded a loss on fair market value of the derivative liability for nine months ended September 30, 2024.
54
Comparison of the Year Ended December 31, 2023 and 2022
The following table summarizes our results of operations and changes for the periods indicated:
| Year ended December 31, | ||||||||||||||||
| 2023 | 2022 | $ Change | % Change | |||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research and development | $ | 510,356 | $ | - | $ | 510,356 | 100 | % | ||||||||
| General and administrative | 366,960 | 11,772 | 355,188 | 3017 | % | |||||||||||
| Total Operating Expenses | 877,316 | 11,772 | 865,544 | 7353 | % | |||||||||||
| LOSS FROM OPERATIONS | (877,316 | ) | (11,772 | ) | (865,544 | ) | 7353 | % | ||||||||
| Interest Expense | 104,487 | - | 104,487 | 100 | % | |||||||||||
| NET LOSS | $ | (981,803 | ) | $ | (11,772 | ) | $ | (970,031 | ) | 8240 | % | |||||
Research and Development Expenses
Research and development expenses for the year ended December 31, 2023 were $510,356. There were no research and development expenses for the year ended December 31, 2022. During 2023, the increase in research and development costs are primarily associated with the execution of the Duke License in 2023. The expenses incurred upon execution of the Duke License of approximately $340,000 were determined to be IPR&D acquired in an asset transaction. These costs were expensed as the technology has not received regulatory approval for marketing and, absent obtaining such approval, has no established alternative future use. The remainder of the increase is primarily related to the initiation of pre-clinical activities in 2023.
General and Administrative Expenses
General and administrative expenses increased by $355,188, or 3,017%, for the year ended December 31, 2023 when compared to the year ended December 31, 2022. The increase was primarily related to increases in consulting costs related to management of the Company as business activities increased along with increases in professional fees for legal and accounting services.
Liquidity and Capital Resources
We have incurred operating losses and experienced negative operating cash flows since our inception, and we anticipate continuing to incur losses for at least the next several years as we continue to develop our product candidates. As of September 30, 2024, we had cash of $115,308 and an accumulated deficit of approximately $3.2 million.
As set forth above, in 2023 and 2024, we raised bridge financing through the offer and sale of $1,500,000 of Bridge Notes. At the closing of the Offering, the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, converted automatically into shares of Common Stock at a conversion price of $3.30 per share, or 501,140 shares of our Common Stock, and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, Pre-Merger Warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of $3.30 or $4.40 per share and with a term of five years.
Based on our current operating plan, we anticipate that our existing cash balance will not be sufficient to fund our operating activities for the next twelve months and, as such, we will need to obtain additional funding. We plan to continue to fund our losses from operations through cash on hand, as well as through future equity offerings, debt financings, or other third-party funding. There can be no assurance that additional funds will be available when needed from any source or, if available, will be available on terms that are acceptable to us. Even if we raise additional capital, we may also be required to modify, delay or abandon some of our plans which could have a material adverse effect on our business, operating results and financial condition and our ability to achieve our intended business objectives. Any of these actions could materially harm our business, results of operations and future prospects.
55
Cash Flows
The following table shows a summary of our cash flows for the periods presented:
| Nine months ended September 30, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| Net cash (used in) provided by: | ||||||||||||||||
| Operating activities | (761,936 | ) | (198,047 | ) | (563,889 | ) | 285 | % | ||||||||
| Financing activities | 872,490 | 398,890 | 473,600 | 119 | % | |||||||||||
| Net increase in cash during the periods | 110,554 | 200,843 | (90,289 | ) | 45 | % | ||||||||||
| For the years ended December 31, | ||||||||||||||||
| 2023 | 2022 | $ Change | % Change | |||||||||||||
| Net cash (used in) provided by: | ||||||||||||||||
| Operating activities | (394,186 | ) | 50 | (394,236 | ) | 788472 | % | |||||||||
| Financing activities | 398,890 | - | 398,890 | 100 | % | |||||||||||
| Net increase in cash during the periods | 4,704 | 50 | 4,654 | 9308 | % | |||||||||||
Operating Activities
Net cash used in operating activities was $761,936 for the nine months ended September 30, 2024, compared to $198,047 of cash used in operating activities for the comparable prior year period. The increase of $563,889 is related to increased operating activity in 2024 consisting of a repeat-dose toxicology study that was initiated in late 2023 along with increases in consulting costs in 2024 related to management of the Company with increased business activities.
Net cash used in operating activities for the year ended December 31, 2023 was $394,186 and reflects costs associated with the execution of the Duke License in 2023 and increases in legal and professional fees. For the year ended December 31, 2022, such costs were only $50 related to the initial cash account funding by the Company’s founders.
Financing Activities
Net cash provided by financing activities was $872,490 for the nine months September 30, 2024 related to the issuance of the 2024 Notes in the amount of $1,000,000 offset by debt issuance costs. For the nine months ended September 30, 2023, net cash provided by financing activities was $398,890 related to the issuance of the 2023 Notes in the amount of $500,000 offset by debt issuance costs.
Net cash provided by financing activities was $398,890 for the year ended December 31, 2023 related to the issuance of the 2023 Notes offset by debt issuance costs. There were no financing activities during the year ended December 31, 2022.
56
Indebtedness
In 2023, we entered into Securities Purchase Agreements resulting in issuance of an aggregate of $500,000 of the 2023 Bridge Notes. We incurred $101,110 in debt issuance costs related to the issuance of this debt. For the year ended December 31, 2023, we also recorded accrued interest of $33,615 and amortization expense of the debt issuance costs of $70,872. There were no debt costs for the year ended December 31, 2022.
During the nine months ended September 30, 2024, we entered into Securities Purchase Agreements resulting in the issuance of an aggregate of $1.0 million of the 2024 Bridge Notes. We incurred approximately $128,000 in debt issuance costs related to the issuance of this debt. As of September 30, 2024, we had recorded accrued interest expense of $26,264, amortization of debt issuance costs of $28,070, amortization of discounts related to an embedded derivative liability of $73,091 and a loss on the fair value of derivative liabilities of $8,755.
Funding Requirements
We use our cash primarily to fund research and development expenditures. We expect our research and development expenses to increase as we continue the development of APTN-101. We expect to incur an increase in general and administrative expenses in 2025 primarily related to supporting our increasing research and development activities and becoming a publicly held company with the resulting professional fees, personnel and regulatory compliance related costs. We expect to incur increasing operating losses for the foreseeable future as we continue the preclinical and clinical development of our product candidate. At this time, due to the inherently unpredictable nature of clinical development, we cannot reasonably estimate the costs we will incur and the timelines that will be required to complete development, obtain marketing approval, and commercialize APTN-101 or any future product candidates, if at all. For the same reasons, we are also unable to predict when, if ever, we will generate revenue from product sales or whether, or when, if ever, we may achieve profitability. Clinical and preclinical development timelines, the probability of success, and development costs can differ materially from expectations.
The timing and amount of our operating expenditures will depend largely on:
| ● | the timing, progress and results of our ongoing and planned preclinical and clinical development activities for APTN- 101 in GBM; |
| ● | the scope, progress, results and costs of preclinical development, testing and clinical trials of APTN-101 for any additional indications; |
| ● | the ability of our vendors and third-party service providers to accurately forecast expenses and deliver on expectations; |
| ● | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and |
| ● | the extent to which we acquire or in-license other product candidates and technologies. |
Until such time, if ever, as we can generate substantial revenue from product sales, we expect to fund our operations and capital funding needs through equity and/or debt financing. We may also consider entering into collaboration arrangements or selectively partnering for clinical development and commercialization. The sale of additional equity would result in additional dilution to our shareholders. The incurrence of debt financing would result in debt service obligations and the instruments governing such debt could provide for operating and financing covenants that restrict our operations or our ability to incur additional indebtedness, among other items. If we are not able to secure adequate additional funding, we may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, and/or suspend or curtail planned programs. Any of these actions could materially and adversely affect our business, financial condition and results of operations.
57
Contractual Obligations and Commitments
Duke University License
In January 2023, Private Adaptin entered into the Duke License with Duke University and the National Cancer Institute, under the agency of the U.S. Department of Health and Human Services for an exclusive, world-wide, sub-licensable license to the technology more fully described above, which was assumed by the Company in the Merger. As a component of the Duke License, the Company is obligated to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities. These milestones include initiation of Phase II or Phase III clinical trials, submission of applications for market approval in multiple jurisdictions including the United States, European Union and Japan and the initiation of post-approval commercial sales in the same jurisdictions. Based on an assumption that all milestones related to the current development program are met during the course of the Duke License, these milestone payments would total approximately $11.7 million. As of September 30, 2024, the Company has not met any milestones as defined in the Duke License and, accordingly, has recorded no expense or liability related to such payments.
The Company is also obligated to pay royalties equal to low- to mid- single digit percentages of annual net sales on a country-by-country and product-by-product basis subject to downward adjustment to low single digit percentages of our net annual sales in the event there is no valid claim of a patent for the product, with minimum annual royalty levels established. We also must pay Duke University low to mid-double digit percentages of any sublicensing fees as set forth in the Duke License. The Company has not recorded and does not owe any royalties or sublicensing fees for the nine months ended September 30, 2024.
Off-Balance Sheet Transactions
We did not have during the periods presented, and we do not currently have, any off-balance sheet financing arrangements or any relationships with unconsolidated entities or financial partnerships, such as structured finance or special purpose entities, that were established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Critical Accounting Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates and assumptions reflected in the financial statements relate to and include, but are not limited to, the fair value of common stock and other assumptions used to measure share-based consideration transferred for acquired assets under the Duke License, the fair value of derivative liabilities along with prepaid expenses and accrued liabilities that are measured based on progress toward completion of research and development projects.
Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
JOBS Act Accounting Election
We are an “emerging growth company,” as defined in the JOBS Act. The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to either early adopt or delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to use the extended transition period under the JOBS Act until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
58
MANAGEMENT
Executive Officers and Directors
The following table provides information regarding our executive officers and directors as of February 11, 2025:
| Name | Age | Positions | ||
| Executive Officers | ||||
| Michael J. Roberts, PhD | 55 | Chief Executive Officer and Director | ||
| Simon C. Pedder, PhD | 63 | Executive Chairman and Director | ||
| Timothy L. Maness, CPA | 64 | Chief Financial Officer | ||
| L. Arthur Hewitt, PhD | 71 | Chief Development Officer | ||
| Non-Employee Directors | ||||
| Patrick Gallagher | 59 | Director | ||
| Anthony Zook | 64 | Director | ||
| J. Nick Riehle | 72 | Director |
| (1) | Member of the Audit Committee. |
Executive Officers
Michael J. Roberts, PhD – Chief Executive Officer and Director
Dr. Roberts has been our Chief Executive Officer and a member of our board of directors since February 2025, and was Chief Executive Officer and a director of Private Adaptin from March 2021 until February 2025. He has over 25 years of pharmaceutical research, development, corporate development, and executive experience. He is co-founder and President and CEO of Adaptin Bio and also serves on the board of directors. Prior to Adaptin he was co-founder of Corino Therapeutics and is currently its acting CEO. Dr. Roberts led and ran all activities related to Corino and the development of a disease-modifying drug called CRX-1008, which is used to treat the protein disorder transthyretin amyloidosis. He is a pharmaceutical and biotech consultant and owner of MAC B Consulting. Prior to Corino, Dr. Roberts was VP, Business Development and Corporate Officer of publicly traded Chelsea Therapeutics International, Ltd. He was responsible for business development efforts focused primarily on licensing and M&A with consideration to pipeline management. He led the sale of Chelsea Therapeutics to H. Lundbeck A/S in 2014 for approximately $658 million. Prior to Chelsea Therapeutics, Dr. Roberts was Director of Business Development for their Nektar Therapeutics’ Molecule Engineering technology and completed a number of transactions with large and specialty pharmaceutical companies. Dr. Roberts has completed pharmaceutical transactions valued at over $1B. Prior to this he was Manager of Biopharmaceutical Research at Shearwater Corporation where he led and was successful in the development of preclinical drug candidates from initial stages of research through Phase I clinical study, including inventing the product Movantik™, a treatment for opioid-induced constipation, subsequently licensed to AstraZeneca in a transaction valued at approximately $1 billion. Shearwater was sold to Inhale Therapeutic Systems (now known as Nektar Therapeutics) in 2001 for approximately $200 million. Dr. Roberts obtained his Ph.D. in Materials Science from the University of Alabama and B.S. in Chemical Engineering from Pennsylvania State University. We believe that Dr. Roberts’ experience in the life sciences industry and in the operation of publicly traded companies as an executive qualifies him to serve on our board of directors.
Simon C. Pedder, PhD – Executive Chairman and Director
Dr. Pedder has been our Executive Chairman and a member of our board of directors since February 2025, and was Executive Chairman and a director of Private Adaptin since October 2023. He has a career of over 30 years in drug development and commercialization. He was Chief Executive Officer of Nirogy from November 2021 to November 2022. From December 2016 to November 2021, he had leadership roles as Chief Business and Strategy Officer for Athenex; President and CEO of Cellectar Biosciences from April 2014 to June 2015; President and CEO of Chelsea Therapeutics International, Ltd. from May 2004 to July 2012; and Global Vice President of Oncology Pharma Business and Executive Officer at Hoffmann-LaRoche. Previous positions at Roche included Life Cycle Leader and Global Project Leader of Pegasys/IFN and Head of Hepatitis Franchise. Prior to that, he was Clinical Leader for a number of development compounds at Roche. Dr. Pedder served on the board of directors of Cerecor, Inc. from April 2018 to June 2020, Mateon Therapeutics, Inc. from March 2016 to April 2019 and Delcath Systems, Inc. from November 2017 to April 2019.
59
Early in his career, he was a faculty member in the Department of Pharmacology in College of Medicine at the University of Saskatchewan, where he obtained his Ph.D. in Clinical Pharmacology. During his longstanding career in pharmaceutical development, Dr. Pedder played key roles in the successful development and commercialization of multiple proprietary pharmaceutical products including Tasmar®, Pegasys®, Copegus®, Northera® and Klisyri®. In addition to his Ph.D., Dr. Pedder obtained a Master of Science in Toxicology from Concordia University, a Joint Honors Bachelor degree in Environmental Studies/Biology from the University of Waterloo, and he completed the Roche-sponsored Pharmaceutical Executive Management Program at Columbia Business School. We believe Dr. Pedder’s experience in the life sciences industry and in the operation of publicly traded companies as an executive and board member qualifies him to serve on our board of directors.
Timothy L. Maness, CPA – Chief Financial Officer
Mr. Maness has been our Chief Financial Officer since February 2025, and was Chief Financial Officer of Private Adaptin from September 2024 to February 2025. Since April 2016, he has provided financial consulting services through Adamanteus, LLC. Mr. Maness has been a consultant to multiple publicly held and private-equity-backed life sciences companies, and he has held several senior financial management roles, primarily in software and technology services.
In 2018, Mr. Maness was engaged by Milestone Pharmaceuticals to prepare and lead its initial public offering, serving as its interim CFO, CAO, and Vice President of Finance. Before that, he served as the CFO of Ballantyne Therapeutics. Earlier, during his eight years as the Senior Director of Finance and Corporate Controller at Chelsea Therapeutics International, he was heavily involved in Chelsea’s reverse-public-shell merger to complete its public listing in 2005. Mr. Maness is a noted expert in forensic accounting and internal audit and has served as the internal auditor hired by public company audit committees on several occasions.
Mr. Maness received a B.S. in accounting from the University of North Carolina at Charlotte, and he is licensed as a Certified Public Accountant and as a Chartered Global Management Accountant.
L. Arthur Hewitt, PhD – Chief Development Officer
Dr. Hewitt has been our Chief Development Officer since February 2025, and was Chief Development Officer of Private Adaptin from September 2024 to February 2025. He has worked for approximately 35 years in clinical research and regulatory affairs. From December 2016 to December 2021, he served as a scientific advisor to Amneal Pharmaceuticals and Senior Scientific Advisor – Neurology at Lundbeck Pharmaceuticals. Prior to Lundbeck, Dr. Hewitt was Chief Scientific Officer for Chelsea Therapeutics International, Ltd. from January 2010 to June 2014 and Vice President, Drug Development from May 2004 to June 2014. At Chelsea, he oversaw the clinical development and regulatory approval of Northera™ (droxidopa), a novel therapeutic agent, for the treatment of symptomatic neurogenic orthostatic hypotension, or Neurogenic OH, in patients with primary autonomic failure (Parkinson’s disease, or PD, multiple systems atrophy, or MSA, and pure autonomic failure, or PAF), dopamine β-hydroxylase, or DBH, deficiency and non-diabetic autonomic neuropathy. Prior to Chelsea, Dr. Hewitt served as an independent contractor from January 2003 to May 2004, and as Director of Scientific Affairs at Shearwater Corporation, a drug delivery company, from October 2002 until January 2003.
From July 1991 until November 2000, Dr. Hewitt was Director of Scientific Affairs for Amgen Canada where he oversaw the clinical research and regulatory requirements for a wide variety of proprietary biologic treatments undergoing Phase I, II and III research. Thirteen individual investigational new drug, or IND, research programs were established covering the therapeutic domains of Hematology, Oncology, Neurology, Infectious Disease, and Inflammation. Dr. Hewitt also developed clinical research programs supporting the approval of three products including Neupogen, Stemgen and Infergen and six supplementary approvals. Prior to Amgen, Dr. Hewitt held positions at Jansen Pharmaceuticals and Park-Davis where he developed research programs for multiple neurology and oncology products. Prior to entering the pharmaceutical industry, he was a Lecturer at Concordia University in Montreal, Canada. Dr. Hewitt obtained his Ph.D. in Pharmacology from the University of Montreal. Additionally, Dr. Hewitt received a Master of Science degree in Toxicology and a B.Sc. (Hon) in Comparative Anatomy and Physiology from Concordia University.
60
Non-Employee Directors
Patrick Gallagher
Mr. Gallagher has been a member of our board of directors since February 2025. He has 20 years of healthcare experience including alternative investments, research and marketing in both the public and private markets. Since January 2018, he has served as the Chief Executive Officer and as a member of the board of directors of Voltron Therapeutics, a privately held biotechnology company. Since August 2014, Mr. Gallagher has served as a Managing Director of Laidlaw & Company (UK) Ltd. From January 2018 to September 2024, Mr. Gallagher served as the Chief Executive Officer and a member of the board of directors at PD Theranostics, Inc. He has also served as Treasurer of Aerwave Medical, Inc. since November 2020. Formerly, Mr. Gallagher was a founding partner and Chief Executive Officer of BDR Research Group, LLC (“BDR”), from July 2001 through October 2010. BDR was an independent sell-side research firm specializing in healthcare investing, financing and operations, serving the institutional investing community at large.
Mr. Gallagher served as VP of Business Development and Investor Relations as well as a strategic consultant for Kinex Pharmaceuticals, a biotechnology firm focused on next-generation therapies in oncology and immunology (now traded on NASDAQ: ATNX). He also served as an advisor to CHD Biosciences, a novel antimicrobial company from July 2012 through August 2014. Mr. Gallagher has served on the boards of directors of BioSig Technologies, Inc., NASDAQ-listed a medical device company that is developing a proprietary technology platform in the electrophysiology space since July 2014 Cingulate Therapeutics, a therapeutics company with a novel drug delivery platform since January 2014; Evermore Global, a global special situations money manager since June 2015; and Algorithm Sciences, Inc. since May 2019. Since September 2014, Mr. Gallagher has served as a Managing Partner at Laidlaw Venture Partners dba Laidlaw & Company (UK) Ltd. and is part of the Investment Banking Healthcare Team. Mr. Gallagher also is a member of Lucius Partners.
Mr. Gallagher earned his Master in Business Administration from Penn State University and his Bachelor of Science in Finance from the University of Vermont. We believe that Mr. Gallagher’s extensive experience in the life sciences industry qualifies him to serve on our board of directors.
Anthony Zook
Mr. Zook has been a member of our board of directors since February 2025. He currently is a member of Lucius Partners. Since July 2020, Mr. Zook has served as a director of BioSig Technologies, Inc. (Nasdaq: BSGM). From May 2023 to December 2024, Mr. Zook was a director of NeoGenomics (Nasdaq: NEO), an oncology laboratory service company where he also served as the chair of the compensation committee. From January 2022 to December 2024, he also served as Chairman to Voltron Therapeutics and Algorithm Sciences, respectively. Mr. Zook was executive vice president of global commercial operations of AstraZeneca Plc from 2010 until 2012. He also served as president and chief executive officer of the North American division of AstraZeneca Plc from 2006 until 2009, and president of Medimmune, the company’s wholly owned biologics division from 2009 until 2010. Mr. Zook previously served as a member of the board of directors of AltheRx from 2013-2014, InHibikase in 2014, Rib-X Pharmaceuticals in 2009, the National Pharmaceutical Council from 2007-2009, PhRMA from 2011-2012, the Pennsylvania Division of the American Cancer Society from 2005-2007 and his alma mater, Frostburg State University from 2016-2018 and re-joined in 2021, where he earned a B.S. degree. Mr. Zook also earned an A.A. degree in chemical engineering from Pennsylvania State University. We believe Mr. Zook’s extensive commercialization experience and expertise in executive leadership qualify him to serve on our board of directors.
61
J. Nick Riehle
Mr. Riehle has been a member of our board of directors since February 2025. He has over 25 years of business and management experience with both large companies and start-up ventures, and nearly 20 years of that has been as the chief financial officer of pharmaceutical companies, including taking these same companies public. Mr. Riehle formerly served as the CFO of Athenex, Inc., managing all aspects of the accounting, treasury, IT, HR, and legal functions and providing support to the company when it went public in June 2017. Prior to this, he was a freelance contractor for companies seeking his expertise in financing and related support. From 2004 – 2014, Mr. Riehle was the CFO of Chelsea Therapeutics International, Ltd. He managed all aspects of the accounting, treasury, facilities, IT, HR, IR and legal functions, including supporting the company in over $300 million in public and private equity financings and being actively involved in the sale of the company to H. Lundbeck A/S in 2014 for $658 million. Prior to Chelsea Therapeutics, Mr. Riehle was the CFO for HAHT Commerce, Inc., managing all aspects of treasury, accounting, IT, legal and inventory administration, as well as having significant involvement in sales administration, services management, HR, facilities and related operational activities, including supporting the company in transactions involving over $60 million in venture financing. Prior to HAHT Commerce, Mr. Riehle held various positions in Canada, the United States and Asia with Nortel Networks, including financial planning and analysis, accounting, marketing and sales administration, government relations, and general administration.
Mr. Riehle earned his Bachelor of Commerce from McGill University, his MBA from York University and is a Certified Management Accountant (CMA). We believe that Mr. Riehle’s experience in the life sciences industry qualifies and in the operation of publicly traded companies as a senior financial executive qualifies him to serve on our board of directors.
Corporate Governance
Appointment of Officers
Our executive officers are appointed by, and serve at the discretion of, our board of directors provided, however, that the board of directors may empower the Chief Executive Officer of the Company to appoint any officer other than the Executive Chairperson, the Chief Executive Officer, the President, the Chief Financial Officer or the Treasurer.
Board of Directors Composition
Our board of directors currently consists of five members. Dr. Roberts, Dr. Pedder, Mr. Gallagher, Mr. Zook, and Mr. Riehle have been designated to serve as members of our board of directors.
Each of our current directors will continue to serve until the election and qualification of his successor, or his earlier death, resignation, disqualification or removal.
Director Independence
Our securities are not listed on a national securities exchange or on any inter-dealer quotation system that has a requirement that a majority of directors be independent. We evaluate independence by the standards for director independence set forth in the Nasdaq Marketplace Rules. Under such rules, our board of directors has determined that all members of the board of directors except for Dr. Roberts and Dr. Pedder are independent directors under Nasdaq Listing Rule 5605(a)(2). In making such independence determination, our board of directors considered the relationships that each non-employee director has with us and all other facts and circumstances that our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Under Nasdaq Marketplace Rules, a director only qualifies as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
62
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (i) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries. We intend to satisfy the audit committee independence requirements of Rule 10A-3 as of the time we list on a national securities exchange.
Our board of directors has undertaken a review of the independence of each director and considered whether each director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. As a result of this review, our board of directors determined that Messrs. Gallagher, Riehle and Zook are “independent directors” as defined under the applicable rules and regulations of the SEC and the listing requirements and rules of Nasdaq. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and current and prior relationships as they may relate to us and our management, including the beneficial ownership of our capital stock by each non-employee director and the transactions involving them described in the section titled “Certain Relationships and Related Party Transactions.”
Family Relationships
There are no family relationships by between or among the members of the board of directors or other executive officers of the Company.
Legal Proceedings
None of the Company’s directors or executive officers are involved in any legal proceedings described in Item 401(f) of SEC Regulation S-K.
Committee of the Board of Directors
Our board of directors has an Audit Committee, which has the composition and responsibilities described below. Members serve on the Audit Committee until their resignation or until otherwise determined by our board of directors.
Audit Committee
The members of our Audit Committee are Messrs. Gallagher, Riehle, and Zook. Mr. Riehle serves as the Chair of this committee. The board of directors has determined that Mr. Riehle is “independent” under the Nasdaq Marketplace Rules and standards established by the SEC rules regarding audit committee members as set forth above.
The Audit Committee oversees on behalf of the board of directors (a) the conduct of the Company’s accounting and financial reporting processes, the audits of our financial statements and the integrity of the Company’s audited financial statements and other financial reports; (b) the performance of the Company’s internal accounting, internal auditing, and financial controls function; (c) the engagement, replacement, compensation, qualifications, independence and performance of our independent auditors, and (d) the portions of our Code of Conduct and Ethics and related policies regarding our accounting, internal accounting controls or auditing matters. The Audit Committee also reviews and approves or disapproves related party transactions identified in Item 404 of SEC Regulation S-K and makes recommendations to the full board of directors regarding the same.
The Audit Committee meets privately with our independent registered public accounting firm from time to time, and such firm has unrestricted access to, and reports directly to, the Audit Committee.
63
CODE OF CONDUCT AND ETHICS
In connection with the Merger, our board of directors adopted a new Code of Conduct and Ethics (the “Code of Ethics”), which applies to all directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions) and employees.
The Code of Ethics addresses, among other matters, conflicts of interest and corporate opportunities, fair dealing, record- keeping and public disclosures, compliance with laws and corporate policies, confidentiality and corporate assets, and reporting and consequences of violations. The provisions of the Code of Ethics are intended to reflect current best practices and enhance the Company’s personnel’s understanding of the Company’s standards of ethical business practices, promote awareness of ethical issues that may be encountered in carrying out an employee’s or director’s responsibilities and improve clarity as to how to address ethical issues that may arise.
If any substantive amendments are made to our Code of Ethics, or if any waiver (including any implicit waiver) of any provision of the Code of Ethics is granted that is required to be disclosed under the rules of the SEC, such amendment or waiver will be disclosed on our website, or, if required, in a current report on Form 8-K.
The newly adopted Code of Ethics did not result in any explicit or implicit waiver of any prior code of conduct or ethics.
compensation of directors and executive officers
Information with respect to the Company’s directors and executive officers is described in the section titled “Management”.
Director Compensation
The Company did not pay any compensation to its directors since inception through September 30, 2024. Following the Merger, the Company intends to provide its non-employee directors with annual cash compensation of $25,000. The chair of the Audit Committee will receive an additional $10,000 in annual cash compensation. Non-employee directors will be eligible to receive such equity grants or awards as may be approved by the board in its discretion. The board of directors intends to grant each non-employee director equity awards equal to 0.20% of the Company’s fully diluted shares outstanding at the time of grant. Messrs. Gallagher and Zook have waived any compensation for their services as directors of the Company. Drs. Roberts and Pedder will be employees and executive officers of the Company and will not be separately compensated for the service as directors.
Executive Officer Compensation
Throughout this section, unless otherwise noted, “we,” “us,” “our,” “Company” and similar terms refer to Private Adaptin prior to the closing of the Merger, and to the Company and its subsidiaries after the closing of the Merger.
This section discusses the material components of the executive compensation program for the Company’s named executive officers who appear in the “2024 Summary Compensation Table” below. In 2024, the “named executive officers” and their positions with the Company were as follows:
| ● | Michael J. Roberts: President and Chief Executive Officer |
| ● | Simon C. Pedder: Executive Chairman |
| ● | Timothy L. Maness: Chief Financial Officer |
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from the currently planned programs summarized in this discussion.
64
2024 and 2023 Summary Compensation Table
The following table sets forth information concerning the compensation of the named executive officers for the Company’s most recent fiscal year.
| Name and principal position | Year | Salary | Bonus | Stock awards | Option awards | All other compensation | Total | |||||||||||||||||||||
| Simon
C. Pedder, PhD Executive Chairman |
2024 | -- | -- | -- | -- | $ | 40,000 | (1) | $ | 40,000 | ||||||||||||||||||
| 2023 | -- | -- | -- | -- | $ | 10,000 | (1) | $ | 10,000 | |||||||||||||||||||
| Michael
J. Roberts, PhD Chief Executive Officer |
2024 | -- | -- | -- | -- | $ | 60,000 | (2) | $ | 60,000 | ||||||||||||||||||
| 2023 | -- | -- | -- | -- | $ | 15,000 | (2) | $ | 15,000 | |||||||||||||||||||
| Timothy
L. Maness, CPA Chief Financial Officer |
2024 | -- | -- | -- | -- | $ | 20,550 | (3) | $ | 20,550 | ||||||||||||||||||
| 2023 | -- | -- | -- | -- | $ | 34,200 | (3) | $ | 34,200 | |||||||||||||||||||
| (1) | Represents amount paid to Dr. Pedder pursuant to the Compensation Agreement as described below. |
| (2) | Represents amount paid to MAC B Consulting LLC, which is owned by Dr. Roberts, pursuant to the Compensation Agreement as described below. |
| (3) | Represents amount paid to Adamanteus LLC, which is owned by Mr. Maness, pursuant to the Consulting Agreement as described below. |
Outstanding Equity Awards at Fiscal Year-End
As of the end of our most recently completed fiscal year, none of our named executive officers held any outstanding equity awards.
Pre-Merger Agreements with Named Executive Officers
We are currently party to compensation agreements with Dr. Pedder and an entity owned by Dr. Roberts, as well as a consulting agreement with an entity owned by Mr. Maness. Descriptions of these agreements appear below. Upon closing of the Merger, these agreements were terminated and replaced by employment agreements with each of Dr. Roberts, Dr. Pedder, and Mr. Maness. See “Post-Merger Employment Agreements with Named Executive Officers” below.
Compensation Agreement – MAC B Consulting LLC. On October 1, 2023, the Company and MAC B Consulting LLC, d/b/a Michael J. Roberts entered into a compensation agreement (the “Roberts Compensation Agreement”). Pursuant to the Roberts Compensation Agreement, Dr. Roberts provides business and pharmaceutical development related services to the Company. The Roberts Compensation Agreement may be terminated by the Company or Dr. Roberts upon 30 days prior written notice to the other party. Pursuant to the Roberts Compensation Agreement, Dr. Roberts is entitled to receive cash compensation at a rate of $15,000 per month so long as the Company has sufficient funding.
65
Compensation Agreement – Simon C. Pedder. On October 1, 2023, the Company and Dr. Pedder entered into a compensation agreement (the “Pedder Compensation Agreement”). Pursuant to the Pedder Compensation Agreement, Dr. Pedder provides business and pharmaceutical development related services to the Company. The Pedder Compensation Agreement may be terminated by the Company or Dr. Pedder upon 30 days prior written notice to the other party. Pursuant to the Pedder Compensation Agreement, Dr. Pedder is entitled to receive cash compensation at a rate of $10,000 per month so long as the Company has sufficient funding.
Consulting Agreement – Adamanteus LLC. On May 31, 2023, the Company and Adamanteus LLC, d/b/a Timothy Maness entered into a consulting agreement (the “Maness Consulting Agreement”). Pursuant to the Maness Consulting Agreement, Mr. Maness provides finance and accounting services to the Company. The Maness Consulting Agreement was for an initial term of six months and has continued for subsequent six-month terms in accordance with the terms of the Maness Consulting Agreement. The Maness Consulting Agreement may be terminated by the Company or Mr. Maness upon 30 days prior written notice to the other party. Pursuant to the Maness Consulting Agreement, Mr. Maness is entitled to receive cash compensation at a rate of $300 per hour.
Post-Merger Employment Agreements with Named Executive Officers
We entered into executive employment agreements with each of Dr. Roberts, Dr. Pedder, and Mr. Maness (each an “Executive” and collectively, the “Executives”), which were effective upon the closing of the Merger. The agreements include customary non-competition, non-solicitation, and confidentiality covenants; establish the Executives’ duties and compensation; and provide for their continued employment with the Company. The discussion that follows summarizes certain other anticipated terms of these agreements.
Term. The initial term of each of the employment agreements commenced upon the closing of the Merger and continue for a term of three years in the case of Dr. Roberts and Dr. Pedder, and two years in the case of Mr. Maness, unless terminated sooner in accordance with the employment agreement (see “Termination” below). After the initial term expires, the employment agreements will automatically renew for successive one-year terms unless either the Company or the Executive provides written notice of their intent not to renew at least 90 days prior to the expiration of the then-current term.
Board Service. In the case of Drs. Roberts and Pedder, the Company will use commercially reasonable efforts to cause these individuals to be elected as members of the Company’s board of directors throughout the terms of their employment agreements.
Base Salary. The Company intends to pay each Executive a base salary at the following annual rates:
Dr. Roberts – $480,000
Dr. Pedder – $240,000
Mr. Maness – $240,000
These salaries will be reviewed by the board of directors from time to time and may be increased in the board of directors’ sole discretion. Salaries may not be reduced except in connection with an across-the-board reduction of executive-level salaries in which the Executive will not be subject to a greater reduction, on a percentage basis, than any other executive- level employee.
Bonus. For each calendar year, the Executives will be eligible to receive an annual bonus based on the following target amounts of each Executive’s base salary:
Dr. Roberts – 50%
Dr. Pedder – 30%
Mr. Maness – 30%
The actual amount of each annual bonus will depend on the level of achievement of the Company’s corporate objectives and the Executive’s individual objectives, in each case, as established by the board for the calendar year with respect to which such annual bonus relates. The annual bonus for a calendar year, to the extent earned, will be paid in a lump sum in the following calendar year. The annual bonus will not be deemed earned until the date that it is paid. Accordingly, in order for the Executive to receive an annual bonus, the Executive must be actively employed by the Company at the time of such payment.
66
Equity Compensation. Each Executive will be eligible to receive such equity grants or awards as may be approved by the board of directors in its discretion.
Subject to approval by the board of directors and the terms of the Company’s equity compensation plan then in place, in the event the Company issues additional securities raising aggregate funds of $10,000,000 (in one or more transactions), occurring, if at all, within two years following the Merger (the “Additional Financing Period”), the Company will grant each Executive options to purchase a number of shares of Common Stock of the Company (the “Anti-Dilution Options”) sufficient to ensure that their respective ownership immediately following the Additional Financing Period, on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable) is equal to their respective ownership immediately following the Merger, as determined on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable). The per share exercise price of the Anti-Dilution Options will be equal to the fair market value of a share of the Company’s Common Stock on the date of grant, as determined by the board of directors. The Anti-Dilution Options, if any, will become exercisable in four equal annual installments, in each case subject to the continued employment of each Executive with the Company on the date each such vesting milestone is achieved, and will be subject to the terms of the Company’s equity incentive plan then in place and a related option grant agreement to be entered between Executive and the Company.
Other Benefits. Dr. Roberts and Dr. Pedder will each be entitled to no less than 25 paid vacation days per year. Mr. Maness will be entitled to no less than 20 paid vacation days per year. The Executives will also be entitled to such other benefits, and to participate in such benefit plans, as are generally made available to similarly situated senior executive employees of the Company. The Company will also reimburse the Executives for all reasonable business expenses they incur in connection with the performance of their duties.
Termination. The employment agreements may be terminated in the following circumstances:
| ● | Automatically effective upon the Executive’s death. |
| ● | By the Company upon notice to the Executive in the event of the Executive’s disability. “Disability” means the inability of the Executive to perform the essential functions of their job for 90 consecutive days or for 120 days in any one-year period due to the condition of the Executive’s physical, mental, or emotional health. |
| ● | By the Company for cause. “Cause” means the Executive’s (i) fraud, embezzlement or misappropriation with respect to the Company; (ii) willful or grossly negligent misconduct that has or may reasonably be expected to have a material adverse effect on the property, business, or reputation of the Company; (iii) material breach of their employment agreement; (iv) willful failure or refusal to perform their material duties or willful failure to follow any specific lawful instructions of the board of directors (in the case of Dr. Roberts and Dr. Pedder) or the chief executive officer (in the case of Mr. Maness); (v) conviction or plea of nolo contendere in respect of a felony or of a misdemeanor involving moral turpitude; or (vi) material failure to comply with the Company’s workplace rules, policies, or procedures. |
| ● | By the Company for any reason other than for cause or the Executive’s disability. |
| ● | By the Executive for good reason. “Good reason” means the occurrence of any of the following events without the Executive’s written consent: (i) the Company’s requiring the Executive to be based at any office or location more than 25 miles from their principal work location, except for travel reasonably required in the performance of the Executive’s responsibilities to the Company; (ii) a material reduction of the Executive’s base salary not in compliance with their employment agreement; (iii) a material diminution of the Executive’s authority, duties, or responsibilities; or (iv) the Company’s material breach of the Executive’s employment agreement. The Company will have an opportunity to cure any such conditions following notice by the Executive. |
| ● | By the Executive upon 30 days’ written notice to the Company at any time for any reason. |
67
Separation Benefits Not in Connection with a Change in Control. If the Company terminates the Executive’s employment without cause or if the Executive resigns for good reason, in either case not in connection with a change in control of the Company, then the Executive will be entitled to the following benefits:
| ● | 24 months of then-current base salary in the case of Dr. Roberts and Dr. Pedder, and 12 months of then-current base salary in the case of Mr. Maness. |
| ● | Continuation of health insurance coverage for the Executive and their family for 18 months in the case of Dr. Roberts and Dr. Pedder, and 12 months in the case or Mr. Maness, or until the Executive becomes eligible for coverage under another employer’s plan. |
The Company’s obligation to provide such benefits is conditioned upon the Executive executing, and not revoking, a release of claims in a form acceptable to the Company.
Separation Benefits in Connection with a Change in Control. If the Company terminates the Executive’s employment without cause, or if the Executive resigns for good reason, in either case at the time of, or within six months following a change in control of the Company, then the Executive will be entitled to the following benefits:
| ● | 24 months of then-current base salary in the case of Dr. Roberts and Dr. Pedder, and 12 months of then- current base salary in the case of Mr. Maness. |
| ● | Accelerated vesting of all equity awards such that all awards are deemed to have been vested as of the date of termination. |
| ● | Continuation of health insurance coverage for the Executive and their family for 18 months in the case of Dr. Roberts and Dr. Pedder, and 12 months in the case or Mr. Maness, or until the Executive becomes eligible for coverage under another employer’s plan. |
The Company’s obligation to provide such benefits is conditioned upon the Executive executing, and not revoking, a release of claims in a form acceptable to the Company.
A “change in control” means the occurrence of any of the following events:
| ● | Any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Exchange Act) but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular qualified participant of any equity incentive plan of the Company (a “Participant”), the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (i) the combined voting power of the Company’s then-outstanding securities; or (ii) the Company’s then-outstanding equity securities (in either such case other than as a result of an acquisition of securities directly from the Company). |
| ● | Any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own, directly or indirectly, equity securities representing in the aggregate 50% or more of the combined voting power of the securities of the entity issuing cash or securities in the consolidation or merger (or of its ultimate parent entity, if any). |
| ● | Any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership of the Company immediately prior to such sale. |
| ● | The members of the board at the beginning of any consecutive 24-calendar-month period (the “Incumbent Directors”) cease for any reason other than due to death to constitute at least a majority of the members of the board; provided that any director whose election, or nomination for election by the Company’s stockholders, was approved or ratified by a vote of at least a majority of the members of the board of directors then still in office who were members of the board of directors at the beginning of such 24-calendar-month period, will be deemed to be an Incumbent Director. |
68
Indemnification. The Company will indemnify each Executive and hold them harmless in connection with the defense of any lawsuit or other claim to which they are made a party by reason of being an officer, director, or employee of the Company, to the fullest extent permitted by Delaware law. This indemnification obligation does not extend to claims arising out of the Executives’ willful misconduct. In addition, the Company will use commercially reasonable efforts to maintain directors’ and officers’ liability insurance for each Executive for acts and omissions occurring during Executive’s employment with the Company.
Description of the 2025 Equity Incentive Plan
Following is a summary of the principal features of the Company’s 2025 Equity Incentive Plan, which we refer to as the 2025 Plan.
Key Provisions
Following are the key provisions of the 2025 Plan:
| Provisions of the 2025 Plan | Description | ||
| Share Reserve: | ● | As of the date of this Report, up to 2,196,390 shares of Common Stock are reserved under the 2025 Plan. | |
| ● | For a period of ten years commencing on February 11, 2025 and ending on (and including) February 11, 2035, the share reserve under the 2025 Plan will be increased by an amount equal to the lesser of (i) 4% of the number of shares outstanding as of December 31 of the immediately preceding calendar year or (ii) such lesser number of shares as determined by the board of directors prior to January 1 of a particular calendar year. | ||
| ● | The reserved shares will be reduced (i) by one share for each share granted pursuant to awards awarded under the 2025 Plan, and (ii) to the extent cash is delivered in lieu of shares of Common Stock upon the exercise of a stock appreciation right, we will be deemed to have issued the number of shares of Common Stock which it was entitled to issue upon such exercise. | ||
| Award Types: | ● | Incentive and nonstatutory stock options | |
| ● | Stock appreciation rights (“SARs”) | ||
| ● | Restricted stock awards | ||
| ● | Restricted stock unit awards (“RSUs”) | ||
| ● | Dividend equivalent rights | ||
| Vesting: | Determined by our board of directors or a committee designated by our board of directors. | ||
| Repricing: | Repricing of outstanding stock awards is not permitted without the approval of our stockholders, except for certain proportionate capitalization adjustments as set forth in the 2025 Plan. | ||
| Termination Date: | February 11, 2035 | ||
Administration
The 2025 Plan will be administered by our board of directors or a committee designated by our board of directors. With respect to grants of awards to our officers or directors, the 2025 Plan will be administered by our board of directors or a designated committee in a manner that permits such grants and related transactions to be exempt from Section 16(b) of the Exchange Act. The plan administrator will have the full authority to select recipients of the grants, determine the extent of the grants, establish additional terms, conditions, rules, or procedures to accommodate rules or laws of applicable non-United States jurisdictions, adjust awards, and to take any other action deemed appropriate; however, no action may be taken that is inconsistent with the terms of the 2025 Plan.
69
To the extent permitted by applicable law, the board of directors may delegate to a committee of one or more officers of the Company the authority to make awards or to take other actions pursuant to the 2025 Plan, but in no event shall an officer be delegated the authority to grant awards to, or amend awards held by, individuals who are subject to Section 16 of the Exchange Act, members of the board of directors, or officers to whom authority to grant or amend awards has been delegated. Any such delegation will be subject to the restrictions and limits that the board of directors specifies at the time of such delegation, and may be rescinded at any time by the board of directors.
Available Shares
Subject to annual adjustment and upon certain corporate transactions or events, the maximum aggregate number of shares of Common Stock which may be issued pursuant to all awards under the 2025 Plan is up to 2,196,390 shares as of the date of this Report.
For a period of ten years commencing on February 11, 2025 and ending on (and including) February 11, 2035, the share reserve described above will be increased by an amount equal to the lesser of (i) 4% of the number of shares outstanding as of December 31 of the immediately preceding calendar year or (ii) such lesser number of shares as determined by the board of directors prior to January 1 of a particular calendar year.
Any shares covered by an award that is forfeited, canceled, or expires will be deemed to have not been issued for purposes of determining the maximum aggregate number of shares which may be issued under the 2025 Plan. Shares that actually have been issued under the 2025 Plan pursuant to an award will not be returned to the 2025 Plan and will not become available for future issuance under the 2025 Plan, other than unvested shares that are forfeited or repurchased by us. In the event any option or other award granted under the 2025 Plan is exercised through the tendering of shares (either actually or through attestation), or in the event tax withholding obligations are satisfied by tendering or withholding shares, any shares so tendered or withheld are not again available for awards under the 2025 Plan. To the extent that cash is delivered in lieu of shares of Common Stock upon the exercise of an SAR, then we will be deemed, for purposes of applying the limitation on the number of shares, to have issued the number of shares of Common Stock which were otherwise issuable upon such exercise. Shares of Common Stock we reacquire on the open market or otherwise using cash proceeds from the exercise of options will not be available for awards under the 2025 Plan.
Dividends
No dividend or dividend equivalent will be paid on any unvested award, although the plan administrator may provide in an award agreement that dividends with respect to unvested portions of awards may accrue and be paid when and if the awards vest and shares are actually issued to the participant.
Eligibility and Types of Awards
The 2025 Plan will permit us to grant stock awards, including stock options, SARs, restricted stock, RSUs, and dividend equivalent rights to our employees, directors, and consultants.
Stock Options
A stock option may be an incentive stock option within the meaning of, and qualifying under, Section 422 of the Code, or a nonstatutory stock option. However, only our employees (or employees of our parent or subsidiaries, if any) may be granted incentive stock options. Incentive and nonstatutory stock options are granted pursuant to option agreements adopted by the plan administrator. The plan administrator will determine the exercise price for a stock option, within the terms and conditions of the 2025 Plan provided that the exercise price of a stock option cannot be less than 100% of the fair market value of our Common Stock on the date of grant (or 110% of the fair market value in the case of certain incentive stock options, as described below). Options granted under the 2025 Plan will become exercisable at the rate specified by the plan administrator.
70
The plan administrator will determine the term of the stock options granted under the 2025 Plan up to a maximum of ten years, except in the case of certain incentive stock options, as described below. Unless the terms of an optionholder’s stock option agreement provide otherwise, if an optionholder’s relationship with us, or any of our affiliates, ceases for any reason other than disability or death, the optionholder may exercise any options otherwise exercisable as of the date of termination, but only during the post-termination exercise period designated in the optionholder’s stock option award agreement. The optionholder’s stock option award agreement may provide that upon the termination of the optionholder’s relationship with us for cause, the optionholder’s right to exercise his or her options will terminate concurrently with the termination of the relationship. If an optionholder’s service relationship with us, or any of our affiliates, ceases due to disability or death, or an optionholder dies within a certain period following cessation of service, the optionholder or his or her estate or person who acquired the right to exercise the award by bequest or inheritance may exercise any vested options for a period of 12 months. The option term may be extended in the event that exercise of the option within the applicable time periods is prohibited by applicable securities laws or such longer period as specified in the stock option award agreement but in no event beyond the expiration of its term.
Acceptable consideration for the purchase of Common Stock issued upon the exercise of a stock option will be determined by the plan administrator and may include (i) cash or check, (ii) a broker-assisted cashless exercise, (iii) the tender of Common Stock previously owned by the optionholder, (iv) a net exercise of the option, (v) past or future services rendered, and (vi) any combination of the foregoing methods of payment.
Unless the plan administrator provides otherwise, awards generally are not transferable, except by will or the laws of descent and distribution. Incentive stock options may be granted only to our employees (or to employees of our parent company and subsidiaries, if any). To the extent that the aggregate fair market value, determined at the time of grant, of shares of our Common Stock with respect to which incentive stock options are exercisable for the first time by an optionholder during any calendar year under any of our equity plans exceeds $100,000, such options will not qualify as incentive stock options and will instead be treated as nonstatutory stock options. A stock option granted to any employee who, at the time of the grant, owns or is deemed to own stock representing more than 10% of the voting power of all classes of our stock (or that of our parent or subsidiaries, if any) may not be an incentive stock option unless (i) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and (ii) the term of the incentive stock option does not exceed five years from the date of grant.
Stock Appreciation Rights
SARs may be granted under the 2025 Plan either concurrently with the grant of an option or alone, without reference to any related stock option. The plan administrator will determine both the number of shares of Common Stock related to each SAR and the exercise price for an SAR, within the terms and conditions of the 2025 Plan, provided that the exercise price of an SAR cannot be less than 100% of the fair market value of the Common Stock subject thereto on the date of grant. In the case of an SAR granted concurrently with a stock option, the number of shares of Common Stock to which the SAR relates will be reduced in the same proportion that the holder of the stock option exercises the related option.
The plan administrator will determine whether to deliver cash in lieu of shares of Common Stock upon the exercise of an SAR. If Common Stock is issued, the number of shares of Common Stock that will be issued upon the exercise of an SAR is determined by dividing (i) the number of shares of Common Stock as to which the SAR is exercised multiplied by the amount of the appreciation in such shares, by (ii) the fair market value of a share of Common Stock on the exercise date.
If the plan administrator elects to pay the holder of the SAR cash in lieu of shares of Common Stock, the holder of the SAR will receive cash equal to the fair market value on the exercise date of any or all of the shares that would otherwise be issuable.
The exercise of an SAR related to a stock option is permissible only to the extent that the stock option is exercisable under the terms of the 2025 Plan on the date of surrender. Any incentive stock option surrendered will be deemed to have been converted into a nonstatutory stock option immediately prior to such surrender.
71
Restricted Stock
Restricted stock awards are awards of shares of our Common Stock that are subject to established terms and conditions. The plan administrator sets the terms of the restricted stock awards, including the size of the restricted stock award, the price (if any) to be paid by the recipient, and the vesting schedule and criteria (which may include continued service to us for a period of time or the achievement of performance criteria). If a participant’s service terminates before the restricted stock is fully vested, all of the unvested shares generally will be forfeited to, or repurchased by, us.
Restricted Stock Units
An RSU is a right to receive stock, cash equal to the value of a share of stock, or other securities, or a combination of the three at the end of a set period or the attainment of performance criteria. No stock is issued at the time of grant. The plan administrator sets the terms of the RSU award, including the size of the RSU award, the consideration (if any) to be paid by the recipient, vesting schedule, and criteria and form (stock or cash) in which the award will be settled. If a participant’s service terminates before the RSU is fully vested, the unvested portion of the RSU award generally will be forfeited to us.
Dividend Equivalent Rights
Dividend equivalent rights entitle the recipient to compensation measured by dividends paid with respect to a specified number of shares of Common Stock. The plan administrator sets the terms of any award of dividend equivalent rights.
Performance-Based Compensation
The 2025 Plan establishes procedures for the Company to grant performance-based awards, meaning awards structured so that they will vest only upon the achievement of performance criteria established by the plan administrator for a specified performance period. Performance criteria may be measured on an absolute (e.g., plan or budget) or relative basis, and may be established on a corporate-wide basis or with respect to one or more business units, divisions, subsidiaries or business segments, or may be established on an individual basis. Relative performance may be measured against a group of peer companies, a financial market index or other acceptable objective and quantifiable indices. The plan administrator will have the discretion to adjust the minimum level of achievement required for achievement of performance awards if the plan administrator determines that a change in our business, operations, corporate structure or capital structure, the manner in which we conduct our business, or other events or circumstances render the performance objectives unsuitable. The plan administrator will also have the discretion to adjust the performance objectives for other material events not originally contemplated when the performance objectives were established, such as extraordinary gains and losses, the effect of changes in accounting standards or principles, acquisitions or divestitures, changes in tax rules or regulations, capital transactions, restructuring, nonrecurring gains or losses or other unusual items.
The business measures that may be used to establish the performance criteria may include one of, or combination of, the following:
| ● | Net earnings or net income (before or after taxes); |
| ● | Earnings per share; |
| ● | Net sales growth; |
| ● | Net operating profit; |
| ● | Return measures (including, but not limited to, return on assets, capital, equity, or sales); |
| ● | Cash flow (including, but not limited to, operating cash flow, free cash flow, and cash flow return on capital); |
| ● | Cash flow per share; |
| ● | Earnings before or after taxes, interest, depreciation, and/or amortization; |
| ● | Gross or operating margins; |
72
| ● | Productivity ratios; |
| ● | Share price (including, but not limited to, growth measures and total stockholder return); |
| ● | Expense targets or ratios; |
| ● | Charge-off levels; |
| ● | Improvement in or attainment of revenue levels; |
| ● | Operating efficiency; |
| ● | Operating expenses; |
| ● | Economic value added; |
| ● | Improvement in or attainment of expense levels; |
| ● | Improvement in or attainment of working capital levels; |
| ● | Debt reduction; |
| ● | Capital targets; |
| ● | Consummation of acquisitions, dispositions, projects, or other specific events or transactions; or |
| ● | Other significant business milestones. |
Corporate Transactions
Effective upon the consummation of a corporate transaction, all outstanding awards under the 2025 Plan will terminate unless they are assumed in connection with the corporate transaction.
The plan administrator has the authority to determine, before or at the time of any corporate transaction, the impact that the corporate transaction will have on outstanding awards under the 2025 Plan. For example, the plan administrator may determine that (i) awards will vest and become exercisable, or that other restrictions on such awards will lapse, (ii) awards will be assumed by the surviving corporation in the corporate transaction or replaced with awards that have substantially equivalent terms, (iii) participants will receive a payment in satisfaction of outstanding awards, and (iv) in the case of options and SARs, participants will receive a payment in an amount equal to the amount, if any, by which the fair market value of the shares subject to award exceeds the exercise price. The plan administrator is not required to treat all awards in the same way.
Compensation Recovery (Clawback) Policy
All awards under the 2025 Plan will be subject to reduction, cancellation, forfeiture or recoupment to the extent necessary to comply with any applicable compensation recovery, clawback, forfeiture or other similar policy adopted by our board of directors and as in effect from time to time or applicable law. Further, to the extent that a participant receives any amount in excess of the amount that the participant should otherwise have received under the terms of an award for any reason (including, without limitation, by reason of a financial restatement, mistake in calculations or other administrative error), the participant may be required to repay any such excess amount to the Company.
Amendment and Termination
Our board of directors generally may amend, suspend, or terminate the 2025 Plan. However, it may not amend the 2025 Plan without stockholder approval for certain actions, such as an increase in the number of shares reserved under the 2025 Plan, modifications to the provisions of the 2025 Plan regarding the grant of incentive stock options, modifications to the provisions of the 2025 Plan regarding the exercise prices at which shares may be offered pursuant to options, extension of the expiration date of the 2025 Plan, and certain modifications to awards, such as reducing the exercise price per share, canceling and regranting new awards with lower prices per share than the original prices per share of the cancelled awards, or canceling any awards in exchange for cash or the grant of replacement awards with an exercise price that is less than the exercise price of the original awards.
Tax Withholding
The plan administrator may require a participant to satisfy any federal, state, local, or foreign tax withholding obligation relating to a stock award by (i) causing the participant to tender a cash payment, (ii) withholding shares of Common Stock from the shares of Common Stock issued or otherwise issuable to the participant in connection with the award, (iii) delivering to the Company already-owned shares of Common Stock, (iv) selling shares of Common Stock from the shares of Common Stock issued or otherwise issuable to the participant in connection with the award, (v) withholding cash from an award settled in cash or other amounts payable to the participant, and/or (vi) any other means that the plan administrator determines both to comply with applicable laws and be consistent with the purposes of the 2025 Plan.
73
Summary of United States Federal Income Tax Aspects Related to 2025 Plan
The following summary is intended only as a general guide to certain United States federal income tax consequences under current law of participation in the 2025 Plan and does not attempt to describe all possible federal or other tax consequences of such participation or tax consequences based on any participant’s particular circumstances. The summary does not purport to be complete, and it does not address the tax consequences of the participant’s death, any tax laws of any municipality, state or foreign country in which a participant might reside, or any other laws other than United States federal income tax laws. Furthermore, the tax consequences are complex and subject to change, and a participant’s particular situation may be such that some variation of the described rules is applicable. Recipients of awards under the 2025 Plan should consult their own tax advisors to determine the tax consequences to them as a result of their particular circumstances.
Incentive Stock Options
A participant recognizes no taxable income for regular income tax purposes as a result of the grant or exercise of an incentive stock option qualifying under Section 422 of the Code.
If a participant holds stock acquired through exercise of an incentive stock option for more than two years from the date on which the option was granted and more than one year after the date the option was exercised for those shares, any gain or loss on a disposition of those shares (a “qualifying disposition”) will be a long-term capital gain or loss. Upon such a qualifying disposition, we will not be entitled to any income tax deduction.
If a participant disposes of underlying shares within two years after the date of grant of the option or within one year after the date of exercise of the option (a “disqualifying disposition”), the difference between the fair market value of the shares on the option exercise date and the exercise price (not to exceed the gain realized on the sale if the disposition is a transaction with respect to which a loss, if sustained, would be recognized) will be taxed to the participant as ordinary income at the time of disposition. Any gain in excess of that amount will be a capital gain. If a loss is recognized, there will be no ordinary income, and such loss will be a capital loss. To the extent the participant recognizes ordinary income by reason of a disqualifying disposition, generally we will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Internal Revenue Code of 1986, as amended (“the Code”) limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation) to a corresponding income tax deduction in the tax year in which the disqualifying disposition occurs.
The difference between the option exercise price and the fair market value of the shares on the exercise date of an incentive stock option is treated as an adjustment in computing the participant’s alternative minimum taxable income and may subject the participant to alternative minimum tax liability for the year of exercise. Special rules may apply after exercise for (i) sales of the shares in a disqualifying disposition, (ii) basis adjustments for computing alternative minimum taxable income on a subsequent sale of the shares, and (iii) tax credits that may be available to participants subject to the alternative minimum tax.
Nonstatutory Stock Options
Options not qualifying as incentive stock options, along with options expressly designated as nonstatutory stock options, will be nonstatutory stock options having no special tax status. A participant generally recognizes no taxable income upon the grant of such an option so long as (i) the exercise price is not less than the fair market value of the stock on the date of grant, and (ii) the option (and not the underlying stock) at such time does not have a readily ascertainable fair market value (as defined in Treasury Regulations under the Code). Upon exercise of a nonstatutory stock option, the participant normally recognizes ordinary income in the amount of the difference between the option exercise price and the then-fair market value of the shares purchased. If the participant is an employee, such ordinary income amount will be subject to withholding of income and employment taxes. Generally, the Company will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation) to an income tax deduction in the tax year in which such ordinary income is recognized by the participant.
74
Upon the disposition of stock acquired by the exercise of a nonstatutory stock option, any recognized gain or loss, based on the difference between the sale price and the fair market value on the exercise date, will be taxed as capital gain or loss, which will be short-term or long-term gain or loss, depending on the holding period of the stock.
Stock Appreciation Rights
A participant will not normally recognize taxable income upon the receipt of an SAR. Upon the exercise of an SAR, the participant will recognize ordinary income in an amount equal to the excess of the fair market value of the underlying shares of Common Stock on the exercise date over the exercise price. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes. The Company generally will be entitled to a deduction equal to the amount of ordinary income recognized by the participant in connection with the exercise of the SAR (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation).
Restricted Stock
A participant acquiring restricted stock generally will recognize ordinary income equal to the difference between the fair market value of the shares on the “determination date” (as defined below) and their purchase price, if any. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes.
The “determination date” is the date on which the participant acquires the shares unless they are subject to a substantial risk of forfeiture and are not transferable, in which case the determination date is the earliest of (i) the date the shares become transferable, (ii) the date the shares are no longer subject to a substantial risk of forfeiture, or (iii) the date the shares are acquired if the participant makes a timely election under Code Section 83(b). If the shares are subject to a substantial risk of forfeiture and not transferable when issued, the participant may elect, pursuant to Section 83(b) of the Code, to have the date of acquisition be the determination date by filing an election with the Internal Revenue Service, and other provisions, no later than 30 days after the date the shares are acquired.
Upon the taxable disposition of shares acquired pursuant to a restricted stock award, any gain or loss, based on the difference between the sale price and the fair market value on the determination date, will generally be taxed as capital gain or loss; however, for any shares returned to the Company pursuant to a forfeiture provision, a participant’s loss may be computed based only on the purchase price (if any) of the shares and may not take into account any income recognized by reason of a Section 83(b) election. Such gain or loss will be long-term or short- term depending on whether the stock was held for more than one year.
We generally will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax reporting obligation) to a corresponding income tax deduction in the year in which the ordinary income from restricted stock is recognized by the participant.
Restricted Stock Units
A participant will not normally recognize taxable income upon receipt of an RSU award. In general, the participant will recognize ordinary income in the year in which the units vest and are settled in an amount equal to any cash received and/or the fair market value of any nonrestricted shares received. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes. We generally will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax reporting obligation) to an income tax deduction equal to the amount of ordinary income recognized by the participant.
75
Dividend Equivalent Rights
A recipient of dividend equivalent rights generally will recognize ordinary income at the time the dividend equivalent right is paid. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes. We will generally be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax reporting obligation) to an income tax deduction equal to the amount of ordinary income recognized by the participant.
Other Awards
We generally will be entitled to an income tax deduction in connection with an award under the 2025 Plan in an amount equal to the ordinary income realized by the participant at the time the participant recognizes such income (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation). Participants typically are subject to income (and employment) tax and recognize such tax at the time that an award is granted, exercised, vests, or becomes nonforfeitable, unless the award provides for a further deferral.
Section 409A
Section 409A of the Code (“Section 409A”) imposes certain requirements on nonqualified deferred compensation arrangements. Most awards granted under the 2025 Plan will be designed to qualify for an exemption from the requirements of Section 409A. Certain awards under the 2025 Plan, however, may be subject to the requirements of Section 409A in form and in operation. Awards that are subject to Section 409A will generally be designed to meet the conditions under Section 409A for avoiding the adverse tax consequences resulting from a failure to comply with Section 409A. If an award under the 2025 Plan is subject to Section 409A and fails to satisfy the requirements of Section 409A, the recipient of that award may recognize ordinary income on the amounts deferred under the award, to the extent vested, which may be before the compensation is actually or constructively received.
Also, if an award that is subject to Section 409A fails to comply with the requirements of Section 409A, Section 409A imposes an additional 20% tax on the participant’s compensation recognized as ordinary income, as well as interest on such deferred compensation.
Impact of Section 162(m) on Tax Deductibility of Awards Under the 2025 Plan
Section 162(m) of the Code limits the deductibility for federal income tax purposes of certain compensation paid to any of our covered employees in excess of $1 million. For purposes of Section 162(m), the term “covered employee” generally includes our chief executive officer, our chief financial officer, our three other most highly compensated officers, any individual who was a covered employee for any taxable year beginning after December 31, 2016, and, for any taxable year beginning after December 31, 2026, the next five highest-compensated employees. Compensation attributable to awards under the 2025 Plan either on its own or when combined with all other types of compensation received by a covered employee from the Company, may cause this limitation to be exceeded in any particular year. In addition, the Company’s ability to realize the benefit of any tax deductions described above depends on our generation of taxable income as well as the requirement of reasonableness, other limitations on deductions in the Code and the satisfaction of tax reporting obligations.
2025 Plan Benefits
The 2025 Plan does not provide for set benefits or amounts of awards and we have not approved any stock awards that are conditioned on stockholder approval of the 2025 Plan. We have not approved any stock awards under the 2025 Plan in connection with the Merger. All future awards to directors, executive officers, employees and consultants under the 2025 Plan are discretionary and cannot be determined at this time.
76
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions with Lucius Partners and Related Persons
On March 10, 2022, Unite Acquisition issued an aggregate of 5,000,000 shares of Common Stock to its sole stockholder, Lucius Partners, for an aggregate purchase price equal to $500, pursuant to the terms and conditions set forth in the Common Stock Purchase Agreement with Lucius Partners. The Company issued these shares of Common Stock under the exemption from registration provided by Section 4(a)(2) of the Securities Act.
Also on March 10, 2022, Unite Acquisition issued an unsecured promissory note to Lucius Partners, pursuant to which the Company agreed to repay Lucius Partners the sum of any and all amounts that Lucius Partners may advance to the Company on or before the date that the Company consummates a business combination with a private company or reverse takeover transaction or other transaction after which the Company would cease to be a shell company (as defined in Rule 12b-2 under the Exchange Act). The Company has used the proceeds from the note to cover its expenses. Interest did not accrue on the outstanding principal amount of the note except if an Event of Default (as defined in the note) has occurred. In the event of an Event of Default, the entire note shall automatically become due and payable (the “Default Date”), and starting from five days after the Default Date, the interest rate on the note shall accrue at the rate of 18% per annum. As of September 30, 2024 and December 31, 2023, the amount due under the note payable was $137,764 and $81,219, respectively.
Also effective March 10, 2022, Unite Acquisition entered into a services agreement with Lucius Partners, pursuant to which Unite Acquisition paid Lucius Partners a quarterly fee of $1,250 for advisory, accounting, and administrative support services. Unite Acquisition used the office space and equipment of its management under this agreement. This services agreement was terminated in connection with the Merger.
On October 28, 2024, Unite Acquisition issued an unsecured promissory note (the “October 2024 Note”) to Lucius Partners Opportunity Fund, LP (“LPOF”) and received $275,000. The annual interest rate on the promissory note is 12%. The note matures on October 28, 2025, and can be prepaid at any time without penalty. Unite Acquisition used the proceeds to pay off the note to Lucius Partners described above and the director fees owed to Nathan Pereira and other accrued expenses. The general partner of the new lender LPOF is Lucius Capital Partners LLC (“LCP”). The investment manager of LPOF is Lucius Capital Fund Management, LLC (“LCFM”). Lucius Partners, LCP and LCFM have two individuals in common as members. The October 2024 Note was repaid in connection with the Merger and Offering.
Following the closing of the Offering, pursuant to an Advisory Services Agreement dated February 11, 2025 with Lucius Partners, the combined Company will pay to Lucius Partners a cash fee of $180,000 for advisory services during the first year following such closing, and has agreed to pay Lucius Partners for advisory services, in advance for four consecutive three-month periods, commencing on the first day of the month that is the first full month 12 months or more after the closing, a cash fee of $45,000 for one year (such two-year period, the “Advisory Period”). The Advisory Period can be renewed for additional one-year periods upon written request by the Company within 60 days prior to the expiry of any Advisory Period.
Policies and Procedures for Related Person Transactions
Our Audit Committee has the primary responsibility for reviewing and approving or disapproving “related party transactions,” as defined in applicable SEC rules and regulations. As provided in the Audit Committee charter, in approving or rejecting any such transaction, our Audit Committee is to consider the relevant facts and circumstances available and deemed relevant to it, including, among other factors, whether the terms or other aspects of the transaction differ from those that would likely be negotiated with independent third parties.
All of the transactions described in this section were entered into prior to the adoption of the Audit Committee charter. Although we have not had a written policy for the review and approval of transactions with related persons, our board of directors has historically reviewed and approved any transaction where a director or officer had a financial interest, including the transactions described above. Prior to approving such a transaction, the material facts as to the relationship or interest of the relevant related person in the agreement or transaction were disclosed to our board of directors. Our board of directors took this information into account when evaluating the transaction and in determining whether such transaction was fair to us and in the best interest of all our stockholders.
77
POTENTIAL CONFLICTS OF INTEREST
The sole holder of Unite Acquisition prior to the Merger, Lucius Partners, holds 3,250,000 shares of Common Stock after the Merger, which represents 40.2% of the outstanding shares of the combined Company as of the date of this Report. Lucius Partners purchased its shares upon formation of the Company for a nominal price. Matthew Eitner, the Chief Executive Officer of the Placement Agent, James Ahern, the Managing Partner of the Placement Agent, and Patrick Gallagher, a member of our board of directors, a Managing Director of the Placement Agent, are members and managers and/or officers of Lucius Partners, and therefore are indirectly material stakeholders of the Company. Anthony Zook is also a member of the board of directors of the Company and is a member of Lucius Partners. The Placement Agent and/or its designees hold warrants to purchase an aggregate of 270,204 shares of our Common Stock. Therefore, following the Merger, such persons can exert substantial control over matters requiring the approval of our stockholders.
Additionally, Lucius Partners has received, and will continue to receive after the Merger, fees from us for advisory and certain other services to the Company, as described above under “Certain Relationships and Related Party Transactions—Transactions with Lucius Partners and Related Persons.” Lucius has acted as Unite Acquisition’s advisor from inception and has provided certain services to Unite Acquisition including, but not limited to, formation and development work, strategic advisory services, operational support services, legal and accounting referral services, working capital and financial strategy and compliance direction services, including but limited to identifying Private Adaptin as a potential merger candidate and assisting Unite Acquisition in all aspect of its development through the Merger.
See “Risk Factors - Our principal stockholders will have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company” and “Certain Relationships and Related Party Transactions—Transactions with Lucius Partners and Related Persons” above.
78
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information with respect to the beneficial ownership of our Common Stock as of February 11, 2025, immediately following the closing of the Merger and the Offering, by:
| ● | each of our named executive officers; |
| ● | each of our directors; |
| ● | all of our current directors and executive officers as a group; and |
| ● | each person, or group of affiliated persons, who beneficially owned more than 5% of our Common Stock. |
We have determined beneficial ownership in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Except as indicated by the footnotes below, we believe, based on information furnished to us, that the persons and entities named in the table below have sole voting and sole investment power with respect to all shares of Common Stock that they beneficially owned, subject to applicable community property laws.
The percentage of shares beneficially owned is computed on the basis of 8,081,953 shares of Common Stock outstanding as of February 11, 2025, after giving effect to the Merger and the closing of the Offering. Shares of Common Stock that a person has the right to acquire within 60 days of February 11, 2025 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated, the address of each beneficial owner in the table below is 3540 Toringdon Way, Suite 200, #250 Charlotte, NC 28277.
| Name | Shares
of Common Stock Beneficially Owned |
Percentage of Common Stock Beneficially Owned |
||||||
| 5% Stockholders | ||||||||
| Lucius Partners LLC(1) | 3,520,204 | 42.15 | % | |||||
| Directors and Named Executive Officers | ||||||||
| Michael J. Roberts, PhD | 2,159,468 | 26.72 | % | |||||
| Simon C. Pedder, PhD | 928,571 | 11.49 | % | |||||
| Timothy L. Maness, CPA | - | - | ||||||
| L. Arthur Hewitt, PhD | - | - | ||||||
| Patrick Gallagher | - | - | ||||||
| Anthony Zook | - | - | ||||||
| J. Nick Riehle | - | - | ||||||
| All directors and executive officers as a group (7 persons) | 3,088,039 | 38.21 | % | |||||
| (1) | Includes 270,204 shares of our Common Stock issuable upon the exercise of immediately vested warrants the Placement Agent and/or its designees hold as of February 11, 2025. Matthew Eitner, the Chief Executive Officer of the Placement Agent, James Ahern, the Managing Partner of the Placement Agent, and Patrick Gallagher, a Managing Director of the Placement Agent, are managing members, members and/or officers of Lucius Partners. Mr. Eitner, but not Messrs. Ahern and Gallagher, has voting and investment control over securities held by the Placement Agent and Lucius Partners. |
79
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY
AND RELATED STOCKHOLDER MATTERS
Our Common Stock is not listed on a national securities exchange, an over-the-counter market or any other exchange. Therefore, there is no trading market, active or otherwise, for our Common Stock and our Common Stock may never be included for trading on any stock exchange, automated quotation system or any over-the-counter market.
As of the date of this Report, we have 8,081,953 shares of Common Stock outstanding held by 114 stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our board of directors and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the board of directors deems relevant.
Shares Eligible for Future Sale
Prior to the Merger, there has been a limited public market for our Common Stock. Future sales of our Common Stock, including shares issued upon the exercise of options or warrants that we may issue, in the public market after the Merger, or the perception that those sales may occur, could cause the prevailing price for our Common Stock to fall or impair our ability to raise equity capital in the future. As described below, only a limited number of shares of our Common Stock will be available for sale in the public market for a period of several months after consummation of the Merger due to contractual and legal restrictions on resale described below. Future sales of our Common Stock in the public market either before (to the extent permitted) or after restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing price of our Common Stock at such time and our ability to raise equity capital at a time and price we deem appropriate.
Upon the closing of the Offering, we had 8,081,953 shares of Common Stock outstanding, of which our directors and executive officers beneficially own an aggregate of 3,088,039 shares. Of those outstanding shares, no shares of Common Stock are freely tradable, without restriction, as of the date of this Report. No shares issued in connection with the Merger or the Offering can be publicly sold under Rule 144 under the Securities Act until 12 months after the date of filing this Report.
Sale of Restricted Shares
All of the approximately 8,081,953 shares of Common Stock outstanding upon completion of the Merger and the initial Offering are “restricted securities” as such term is defined in Rule 144. These restricted securities were issued and sold by us in private transactions and are eligible for public sale only if registered under the Securities Act or if they qualify for an exemption from registration under the Securities Act, including the exemptions provided by Rule 144, which rules are summarized below.
Rule 144
Pursuant to Rule 144 promulgated under the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from the date on which this Report, reflecting our status as a non-shell company, is filed with the SEC and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Current Reports on Form 8-K. Our stockholders will be forced to hold their shares of our Common Stock for at least the 12-month period after this Report is filed before they are eligible to sell those shares, and even after that 12-month period, sales may not be made under Rule 144 unless we and the selling stockholders are in compliance with other requirements of Rule 144.
80
In general, Rule 144 provides that (i) any of our non-affiliates that has held restricted Common Stock for at least 12 months is thereafter entitled to sell its restricted stock freely and without restriction, provided that we remain compliant and current with our SEC reporting obligations, and (ii) any of our affiliates, which includes our directors, executive officers and other person in control of us, that has held restricted Common Stock for at least 12 months is thereafter entitled to sell its restricted stock subject to the following restrictions: (a) we are compliant and current with our SEC reporting obligations, (b) certain manner of sale provisions are satisfied, (c) a Form 144 is filed with the SEC, and (d) certain volume limitations are satisfied, which limit the sale of shares within any three-month period to a number of shares that does not exceed 1% of the total number of outstanding shares or, if our Common Stock is then listed or quoted for trading on a national securities exchange, then the greater of 1% of the total number of outstanding shares and the average weekly trading volume of our Common Stock during the four calendar weeks preceding the filing of the Form 144 with respect to the sale. A person who has ceased to be an affiliate at least three months immediately preceding the sale and who has owned such shares of Common Stock for at least one year is entitled to sell the shares under Rule 144 without regard to any of the limitations described above.
Regulation S
Regulation S under the Securities Act provides that shares owned by any person may be sold without registration in the United States, provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the United States (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our shares of Common Stock may be sold in some other manner outside the United States without requiring registration in the United States.
Stock Plans
We intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of Common Stock that are outstanding or reserved for issuance under the 2025 Plan. Such registration statement is expected to be filed and become effective as soon as practicable after the consummation of the Merger (but no fewer than 60 days thereafter pursuant to SEC rules that apply to former shell companies). Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
81
DESCRIPTION OF CAPITAL STOCK
The following description summarizes the most important terms of our capital stock following the Merger and Offering. Because it is only a summary, it does not contain all the information that may be important to you and the descriptions herein are qualified by reference to our restated certificate of incorporation and restated bylaws. For a complete description, you should refer to our restated certificate of incorporation and restated bylaws, which are included as exhibits hereto, and to the applicable provisions of Delaware law.
We have authorized capital stock consisting of 50,000,000 shares of Common Stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share.
As of the date of this Report, we had 8,081,953 shares of Common Stock issued and outstanding, warrants exercisable for 2,023,995 shares of Common Stock outstanding, and no shares of preferred stock issued and outstanding. Unless stated otherwise, the following discussion summarizes the term and provisions of our restated certificate of incorporation and our restated bylaws.
Common Stock
Dividend Rights
Subject to applicable law and the rights and preferences, if any, of any holders of any outstanding series of preferred stock, the holders of our Common Stock are entitled to receive dividends if our board of directors, in its discretion, determines to issue dividends and then only at the times and in the amounts that our board of directors may determine, payable either in cash, in property or in shares of capital stock.
Voting Rights
Holders of our Common Stock are entitled to one vote for each share of Common Stock held on all matters submitted to a vote of stockholders. Except as otherwise required by law, holders of Common Stock are not entitled to vote on any amendment to the restated certificate of incorporation (including any certificate of designation relating to any series of preferred stock) that relates solely to the terms of one or more outstanding series of preferred stock if the holders of such affected series are entitled, either separately or together as a class with the holders of one or more other such series, to vote on such amendment pursuant to the restated certificate (including any certificate of designation relating to any series of preferred stock). We have not provided for cumulative voting for the election of directors in our restated certificate of incorporation. Accordingly, holders of a majority of the shares of our Common Stock are able to elect all of our directors.
No Preemptive or Similar Rights
Our Common Stock is not entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions.
Right to Receive Liquidation Distributions
Upon our liquidation, dissolution, or winding-up and after payment in full of all amounts required to be paid to creditors and to any holders of preferred stock having liquidation preferences, if any, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our Common Stock.
Preferred Stock
Our board of directors is authorized, subject to limitations prescribed by Delaware law, to issue preferred stock in one or more series, to establish from time to time the number of shares to be included in each series, and to fix the designation, vesting, powers (including voting powers), preferences, and relative, participating, optional or other rights of the shares of each series and any of its qualifications, limitations, or restrictions, in each case without further vote or action by our stockholders.
82
Our board of directors can also increase or decrease the number of shares of any series of preferred stock, but not below the number of shares of that series then outstanding or above the total number of authorized shares of the class, without any further vote or action by our stockholders. Our board of directors may, without stockholder approval, authorize the issuance of preferred stock with voting or other rights that could adversely affect the voting power or other rights of the holders of our Common Stock and could have anti-takeover effects. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring, or preventing a change in our control or the removal of existing management and might adversely affect the market price of our Common Stock.
Stock Options
As of the date of this Report, we have no outstanding stock options to shares of our Common Stock under the 2025 Plan or otherwise.
Warrants
As of the date of this Report, we had outstanding the following warrants:
| (i) | Common A Warrants to purchase an aggregate of 1,080,814 shares of our Common Stock, with an exercise price of $4.40 per share; |
| (ii) | Common B Warrants to purchase an aggregate of 540,407 shares of our Common Stock, with an exercise price of $6.60 per share; |
| (iii) | Pre-Merger Warrants to purchase an aggregate of 132,570 shares of our Common Stock, with an exercise price of either $3.30 per share or $4.40 per share; and |
| (iv) | Placement Agent Warrants to purchase an aggregate of 270,204 shares of our Common Stock, with an exercise price of $4.40 per share of our Common Stock. |
Registration Rights Agreement
For a description of the Registration Rights Agreement that we entered into in connection with the Merger and the Offering, see “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Registration Rights” above. All descriptions of the Registration Rights Agreement herein are qualified in their entirety by reference to the text thereof filed as Exhibit 10.8 hereto and incorporated herein by reference.
Anti-Takeover Provisions
The provisions of the DGCL, our restated certificate of incorporation, and our restated bylaws could have the effect of delaying, deferring, or discouraging another person from acquiring control of our Company by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These provisions, which are summarized below, are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and encourage persons seeking to acquire control of our Company to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms. However, these provisions may delay, deter or prevent a merger or acquisition of us that a stockholder might consider is in their best interest or in our best interests, including transactions that might result in a premium over the prevailing market price of our Common Stock.
83
Section 203 of the DGCL
We are subject to the provisions of Section 203 of the DGCL regulating corporate takeovers. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner as summarized below. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| ● | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| ● | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding shares owned by persons who are directors and also officers, and employee stock plans in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| ● | at or after the time the stockholder became interested, the business combination was approved by our board and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| ● | any merger or consolidation involving the corporation and the interested stockholder; |
| ● | any sale, transfer, lease, pledge, or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| ● | subject to exceptions, any transaction that results in the issuance of transfer by the corporation of any stock of the corporation to the interested stockholder; |
| ● | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and |
| ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Restated Certificate of Incorporation and Restated Bylaw Provisions
Our restated certificate of incorporation and our restated bylaws include a number of provisions that may have the effect of deterring hostile takeovers, or delaying or preventing changes in control of our management team or changes in our board of directors or our governance or policy, including the following:
| ● | Board of Director Vacancies. Our restated bylaws and restated certificate of incorporation provide, subject to the special rights of the holders of any series of preferred stock to elect directors, that any vacancy on the board of directors may be filled by the affirmative vote of a majority of the directors then in office, even if less than a quorum, or by a sole remaining director, and not by the stockholders, unless (a) the board of directors determines by resolution that any such vacancies or newly created directorships shall be filled by the stockholders or (b) as otherwise provided by law. Any director chosen to fill a vacancy will hold office until the next annual meeting of stockholders and until his or her successor is duly elected and qualified, or until his or her earlier death, resignation, disqualification or removal. In addition, the number of directors constituting the total number of authorized directors is permitted to be set only by a resolution adopted by a majority of the board of directors. These provisions prevent a stockholder from increasing the size of our board of directors and gaining control of our board of directors by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of the board of directors, but promotes continuity of management. |
84
| ● | Supermajority Requirements for Amendments of Our Restated Certificate of Incorporation and Restated Bylaws. Our restated certificate of incorporation provides that the affirmative vote of holders of at least 66 2/3% of our capital stock entitled to vote generally in the election of directors, voting together as a single class, is required to amend certain provisions of our restated certificate of incorporation, including provisions relating to the size of the board of directors, the limitation of personal liability for the board of directors and officers, special meetings, actions by written consent, the choice of forum provision, and designation of our preferred stock. The affirmative vote of holders of at least 66 2/3% of our capital stock entitled to vote generally in the election of directors, voting together as a single class, is required to amend or repeal our restated bylaws, although our restated bylaws may be amended by the approval of a majority of the board of directors. |
| ● | Stockholder Action; Special Meetings of Stockholders. Our restated certificate of incorporation provides that our stockholders may not take action by written consent but may only take action at annual or special meetings of our stockholders. As a result, holders of our capital stock are not able to amend our restated bylaws or remove directors without holding a meeting of our stockholders called in accordance with our restated bylaws. Our restated certificate of incorporation and our restated bylaws provide that special meetings of our stockholders may be called only by the chairperson or executive chairperson of the board of directors, the lead independent director, our chief executive officer or the board of directors acting pursuant to a resolution adopted by a majority of the board of directors. Additionally, only the business as stated in the notice for a special meeting may be considered at a special meeting of stockholders. Therefore, stockholders are both prohibited from calling a special meeting and from raising additional matters for consideration at a special meeting of stockholders. These provisions might delay the ability of our stockholders to force consideration of a proposal or for stockholders to take any action, including the removal of directors. |
| ● | Advance Notice Requirements for Stockholder Proposals and Director Nominations. Our restated bylaws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. Our restated bylaws also specify certain requirements regarding the timing, form and content of a stockholder’s notice. These provisions may preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders. These provisions might also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our Company. |
| ● | No Cumulative Voting. The DGCL provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. Our restated certificate of incorporation and restated bylaws do not provide for cumulative voting. |
| ● | Issuance of Undesignated Preferred Stock. Our board has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by our board of directors. The existence of authorized but unissued shares of preferred stock enables our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest, or otherwise. |
| ● | Choice of Forum. Our restated certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum and to the fullest extent permitted by law, that the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) and any appellate court therefrom, is the sole and exclusive forum for: (a) any derivative action, suit or proceeding brought on behalf of us; (b) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee, or agent of ours; (c) any action, suit or proceeding asserting a claim against us or any current or former director, officer or employee of ours arising out of or pursuant to, or seeking to enforce any right, obligation or remedy under, or to interpret, apply, or determine the validity of, any provision of the DGCL, the restated certificate of incorporation or the restated bylaws (as each may be amended from time to time); (d) any action, suit or proceeding as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware; or (e) any action, suit or proceeding asserting a claim against us or any current or former director, officer or employee of ours governed by the internal affairs doctrine, in all cases subject to the court having personal jurisdiction over the indispensable parties named as defendants. However, such forum selection provisions do not apply to actions, suits or proceedings brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts of the United States have exclusive jurisdiction. The restated certificate of incorporation also provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America is the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. |
85
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all claims brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Accordingly, both state and federal courts have jurisdiction to entertain such claims. As noted above, the restated certificate of incorporation provides that the federal district courts of the United States have exclusive jurisdiction over any action asserting a cause of action arising under the Securities Act. Accordingly, there is uncertainty as to whether a court would enforce such provision. Our stockholders shall not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
Section 27 of the Exchange Act creates exclusive federal jurisdiction over all claims brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As noted above, the restated certificate of incorporation provides that the choice of forum provision does not apply to suits brought to enforce any duty or liability created by the Exchange Act. Accordingly, actions by our stockholders to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder must be brought in federal court. Our stockholders shall not be deemed to have waived our compliance with the federal securities laws and the regulations promulgated thereunder.
Any person or entity purchasing or otherwise acquiring or holding any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum selection provisions in the restated certificate of incorporation.
The choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees or could result in increased costs for our stockholders to bring a claim in the chosen forum, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find the choice of forum provisions contained in the restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
Limitation on Liability and Indemnification of Directors and Officers
The restated bylaws provide that our directors and officers will be indemnified and advanced expenses by us to the fullest extent authorized or permitted by the DGCL as it now exists or may in the future be amended. In addition, the restated certificate of incorporation provides that our directors and officers will not be personally liable to us or our stockholders for monetary damages for breaches of their fiduciary duty as directors or officers to the fullest extent permitted by the DGCL as it now exists or may in the future be amended.
The restated bylaws also permit us to purchase and maintain insurance on behalf of any officer, director, employee or agent of ours for any liability arising out of his or her status as such, regardless of whether the DGCL would permit indemnification.
86
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against our directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against our directors and officers pursuant to these indemnification provisions.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is Vstock Transfer, LLC. The transfer agent’s address is 18 Lafayette Place, Woodmere, NY 11598, and its telephone number is (212) 828-8436.
Stock Quotation
OUR COMMON STOCK IS CURRENTLY NOT LISTED ON A NATIONAL SECURITIES EXCHANGE OR ANY OTHER EXCHANGE, OR QUOTED ON AN OVER THE COUNTER MARKET. FOLLOWING COMPLETION OF THE OFFERING, WE INTEND TO CAUSE OUR COMMON STOCK TO BE QUOTED ON THE OTC MARKETS QB TIER AS SOON AS PRACTICABLE FOLLOWING THE EFFECTIVENESS OF THE REGISTRATION STATEMENT. HOWEVER, WE CANNOT ASSURE YOU THAT WE WILL BE ABLE TO DO SO AND, EVEN IF WE DO SO, THERE CAN BE NO ASSURANCE THAT OUR COMMON STOCK WILL CONTINUE TO BE QUOTED ON THE OTC MARKETS OR QUOTED OR LISTED ON ANY OTHER MARKET OR EXCHANGE, OR THAT AN ACTIVE TRADING MARKET FOR OUR COMMON STOCK WILL DEVELOP OR CONTINUE.
LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we, or any of our officers or directors in their capacity as such, are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
ITEM 3.02 UNREGISTERED SALES OF EQUITY SECURITIES.
The Offering
The information regarding the Bridge Notes, the Pre-Merger Warrants, the securities issued in the Offering and the Placement Agent Warrants set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Bridge Financings” and “—The Offering” and “Description of Capital Stock” is incorporated herein by reference.
On February 11, 2025, in connection with the Offering, we issued 1,080,814 shares of our Common Stock to 95 accredited investors. These transactions were exempt from registration under Section 4(a)(2) of the Securities Act as not involving any public offering or Regulation D promulgated thereunder.
87
Sales of Unregistered Securities of Adaptin
The following list sets forth information as to all securities Private Adaptin sold from its incorporation as Centaur Bio Inc. on March 12, 2021 through immediately prior to the consummation of the Merger, which were not registered under the Securities Act. The following description is historical and has not been adjusted to give effect to the Merger.
| 1. | On March 17, 2021, Private Adaptin issued a total of 1,000 shares of Private Adaptin’s common stock at a price per share of $0.001 to Dr. Roberts for gross proceeds of $1.00. Private Adaptin relied upon the exemption from registration provided by Regulation 4(a)(2) under the Securities Act. |
| 2. | On August 25, 2021, Private Adaptin issued a total of 430 shares of Private Adaptin’s common stock at a price per share of $0.001 to Dr. Pedder for gross proceeds of $0.43. Private Adaptin relied upon the exemption from registration provided by Regulation 4(a)(2) under the Securities Act. |
| 3. | Private Adaptin issued a total of 75 shares of Private Adaptin’s common stock as part of the consideration for the Duke License. Private Adaptin relied upon the exemption from registration provided by Regulation 4(a)(2) under the Securities Act. |
| 4. | From March 2024 to September 2024, Private Adaptin entered into securities purchase agreements with certain accredited investors for the 2024 Bridge Notes, which provided for an aggregate of $1,000,000, 10% secured subordinated convertible promissory notes payable to investors with a term of 12 months from the issuance date. Additionally, the 2024 Bridge Notes, as amended, provided that, upon a qualified offering, the 2024 Bridge Notes, together with all accrued but unpaid interest at the date of conversion, shall automatically be converted into shares of Private Adaptin or Company common stock at a conversion price equal to 75% of the gross offering price to the investors in such qualified offering. The 2024 Bridge Notes were sold pursuant to Regulation D under the Securities Act. See Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Bridge Financings” for additional information on the 2024 Bridge Notes. |
| 5. | From April 2023 to September 2023, Private Adaptin entered into securities purchase agreements with certain accredited investors for the 2023 Bridge Notes, which provided for an aggregate of $500,000, 10% secured subordinated promissory notes payable to investors with a term of 12 months from the issuance date. The 2023 Bridge Notes were exchanged for an aggregate of $500,000 of the Exchange Notes. The Exchange Notes provided that, upon a qualified offering, the Exchange Notes, together with all accrued but unpaid interest at the date of conversion, shall automatically be converted into shares of Private Adaptin or Company common stock at a conversion price equal to 75% of the gross offering price to the investors in such qualified offering. The 2023 Bridge Notes were sold pursuant to Regulation D under the Securities Act. See Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Bridge Financings” for additional information on the 2023 Bridge Notes. |
ITEM 3.03 MATERIAL MODIFICATION TO RIGHTS OF SECURITY HOLDERS.
The information contained in Item 5.03, “Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year” is incorporated herein by reference.
ITEM 4.01 CHANGES IN REGISTRANT’S CERTIFYING ACCOUNTANT.
On February 11, 2025, the Company’s Board of Directors approved the engagement of WithumSmith+Brown, PC (“Withum”) as the Company’s independent registered public accounting firm to audit its financial statements, effective following the filing of its Form 10-K for the fiscal year ended December 31, 2024 (“Form 10-K”). Upon filing of the Company’s Form 10-K, the Company’s current independent registered public accounting firm, KNAV CPA LLP (“KNAV”), will be dismissed.
During the period from March 10, 2022 (inception) to December 31, 2022, the fiscal years ended December 31, 2024 and 2023 and the subsequent interim period through the date of this Report, there were no disagreements with KNAV on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of KNAV, would have caused it to make reference to the subject matter thereof in connection with its report.
KNAV’s report on the Company’s financial statements as of December 31, 2023 and 2022 and for the year ended December 31, 2023, and for the period from March 10, 2022 (inception) through December 31, 2022 did not contain any adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope or accounting principles except for an explanatory paragraph in such report regarding substantial doubt about Company’s ability to continue as a going concern.
88
During the period from March 10, 2022 (inception) to December 31, 2022, the fiscal years ended December 31, 2024 and 2023 and the subsequent interim period through the date of this Report, neither the Company nor anyone acting on its behalf consulted Withum regarding the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements.
We have provided KNAV with a copy of this Report prior to the filing hereof and have requested that KNAV furnish to us a letter addressed to the SEC stating whether KNAV agrees with the statements made by us under this Item 4.01. KNAV has furnished such letter, which letter is filed as Exhibit 16.1 hereto, as required by Item 304(a)(3) of Regulation S-K.
ITEM 5.01 CHANGES IN CONTROL OF REGISTRANT.
The information regarding change of control of the Company in connection with the Merger set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions” is incorporated herein by reference.
ITEM 5.02 DEPARTURE OF DIRECTORS OR PRINCIPAL OFFICERS; ELECTION OF DIRECTORS; APPOINTMENT OF PRINCIPAL OFFICERS; COMPENSATORY ARRANGEMENTS OF CERTAIN OFFICERS.
The information regarding departure and election of our directors and departure and appointment of our principal officers in connection with the Merger set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets— The Merger and Related Transactions” is incorporated herein by reference.
For information regarding the terms of employment of our newly appointed executive officers, see “Compensation of Directors and Executive Officers—Executive Compensation Arrangements” in Item 2.01 of this Report, which description is incorporated herein by reference. For certain biographical, related party and other information regarding our newly appointed executive officers, see the disclosure under the headings “Management” and “Certain Relationships and Related Party Transactions” in Item 2.01 of this Report, which disclosures are incorporated herein by reference.
For information about compensation to our directors, see “Management” in Item 2.01 of this Report, which description is incorporated herein by reference. For information about the committees each director serves on, see “Management—Committee of the Board of Directors” in Item 2.01 of this Report, which description is incorporated herein by reference. There are no arrangements or understandings pursuant to which any of our current directors was appointed as a director. For certain biographical, related party and other information regarding our newly appointed directors, see the disclosure under the headings “Management” and “Certain Relationships and Related Party Transactions” in Item 2.01 of this Report, which disclosures are incorporated herein by reference.
ITEM 5.03 AMENDMENTS TO ARTICLES OF INCORPORATION OR BYLAWS; CHANGE IN FISCAL YEAR.
Amendments to Certificate of Incorporation
Prior to the Merger, Unite Acquisition’s board of directors approved the amendment and restatement of our certificate of incorporation on February 11, 2025, and stockholders holding 100% of the then outstanding shares of our Common Stock approved the amendment and restatement to our certificate of incorporation on February 11, 2025. The information regarding the restated certificate of incorporation in Item 2.01, “Completion of Acquisition or Disposition of Assets— Description of Capital Stock—Anti-Takeover Provisions” is incorporated herein by reference. Our restated certificate of incorporation is filed as Exhibit 3.2 hereto and is incorporated herein by reference.
Amendments to Bylaws
Prior to the Merger, we amended and restated our bylaws in their entirety, to be effective upon closing of the Merger. The information regarding the restated bylaws in Item 2.01, “Completion of Acquisition or Disposition of Assets— Description of Capital Stock—Anti-Takeover Provisions” is incorporated herein by reference. Our restated bylaws are filed as Exhibit 3.3 hereto and is incorporated herein by reference.
89
ITEM 5.05 Amendments to the Registrant’s Code of Ethics, or Waiver of a Provision of the Code of Ethics.
In connection with the Merger, the board of directors adopted a new Code of Ethics, which applies to all directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions) and employees. The information regarding Code of Ethics of the Company is set forth in Item 2.01, “Code of Conduct and Ethics” is incorporated herein by reference. The newly adopted Code of Ethics did not result in any explicit or implicit waiver of any prior code of conduct or ethics. The foregoing description of the Code of Ethics does not purport to be complete and is qualified in its entirety by the full text of the Code of Ethics, a copy of which is attached hereto as Exhibit 14.1 and is incorporated herein by reference.
ITEM 5.06 CHANGE IN SHELL COMPANY STATUS.
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act). As a result of the Merger, we have ceased to be a shell company. The information contained in this Report constitutes the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act.
ITEM 9.01 FINANCIAL STATEMENTS AND EXHIBITS.
| (a) | As a result of the Merger, as described in Item 2.01, the registrant is filing herewith audited financial statements for Private Adaptin as of and for the fiscal years ended December 31, 2023 and 2022 as Exhibit 99.1 to this Report. |
| As a result of the Merger, as described in Item 2.01, the registrant is filing herewith unaudited financial statements for Private Adaptin as of and for the nine-month periods ended September 30, 2024 and 2023 as Exhibit 99.2 to this Report. | |
| (b) | As a result of the Merger, as described in Item 2.01, the registrant is filing herewith unaudited pro forma condensed combined financial statements as of September 30, 2024, for the nine months ended September 30, 2024 and for the year ended December 31, 2023 as Exhibit 99.3 to this Report. |
| (c) | Shell Company Transactions. Reference is made to Items 9.01(a) and 9.01(b) and the exhibits referred to therein, which are incorporated herein by reference. |
| (d) | Exhibits. |
90
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| * | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with Item 601(b)(10) of Regulation S-K. The registrant hereby agrees to furnish supplementally copies of any of the omitted portions of this exhibit to the SEC upon its request. |
| § | Certain exhibits or schedules to this exhibit have been omitted in accordance with Item 601(a)(5) of Regulation S-K. The registrant hereby agrees to furnish supplementally a copy of any omitted exhibit or schedule to the SEC upon its request. |
91
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Adaptin Bio, Inc. | ||
| Date: February 18, 2025 | By: | /s/ Michael J. Roberts |
| Michael J. Roberts | ||
| President and Chief Executive Officer | ||
92